November 7, 2013 — Corindus Vascular Robotics announced the launch of its CorPath One
Stent Program. The goal of the program is to raise awareness of the potential to decrease longitudinal geographic miss (LGM) caused by sub-optimal stent placement, as well as the patient safety and financial benefits that can result from using the CorPath
Vascular Robotic System to place just one stent per lesion in percutenous coronary interventions (PCI). Corindus is offering a $1,000 credit to hospitals that use two or more stents per lesion in qualifying coronary angioplasties performed with the CorPath System.
"No matter how good we are as physicians, we are still human and imprecision in stent placement or stent size selection can occur," said Ronald Caputo, M.D., director, cardiac services and cardiology research, St. Joseph's Hospital, Syracuse, N.Y., current CorPath user and participant, CorPath PRECISE trial conducted in 2011. "Introducing CorPath into our
cath lab has provided substantial benefits to both physicians and patients in terms of our ability to more accurately select the stent size and enhance dexterity in delivering it to a precise location. Ultimately, these capabilities may result in improved outcomes for the patient and an enhanced PCI procedure for the physician."
Up to 47 percent of stents are not optimally positioned. In an attempt to avoid inferior clinical outcomes due to LGM, the CorPath System was designed to bring robotic precision to coronary angioplasty procedures and stent positioning.
"Although manual PCIs are widely accepted in cath labs across the country, robotic-assisted PCIs could provide some notable advantages over the current care paradigm," said Venkat Rajan, medical device analyst, Frost & Sullivan. "As seen in other procedures, the introduction of robotic-assisted systems has often afforded specialists unparalleled control and precision when attempting critical life-saving procedures."
PCI procedures performed in the United States use more than 1.2 stents per lesion, according to the national average. A number of factors can cause additional stenting, including challenges in visualizing the lesion, improper stent selection due to inaccurate lesion length assessment and misplacement of the stent during device delivery. In cases where more than one stent is used, the hospital's procedure profitability is reduced substantially.
"The CorPath One Stent Program is evidence that Corindus stands behind their product and its potential to avoid excess stenting and provide significant financial and safety benefits to the hospital and the patient," said John Cannizzaro, M.D., administrator, cardiovascular service line, St. Joseph's Hospital.
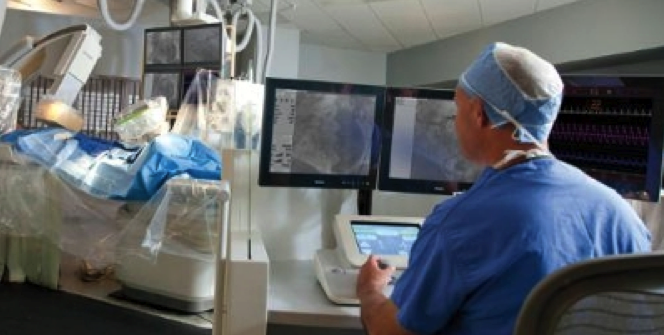
The CorPath System is the first and only U.S. Food and Drug Administration (FDA)-cleared technology that enables precise, robotic-assisted angioplasties to open arteries and restore blood flow in patients with coronary artery disease (CAD). During a CorPath angioplasty procedure, the interventional cardiologist sits in the radiation shielded interventional cockpit. Using robotic precision, the interventional cardiologist advances stents and guidewires via a joystick with millimeter-by-millimeter precision. CorPath may improve clinical outcomes by enabling precise measurement of the anatomy, which could potentially lead to better stent placements.
Demonstrations of the CorPath System took place at the 25th annual Cardiovascular Therapeutics Annual Conference scientific symposium (TCT 2013) in San Francisco.
For more information: www.corindus.com