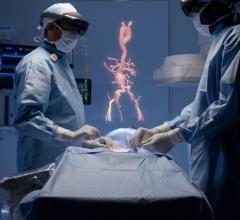
August 1, 2019 — Dassault Systèmes announced the five-year extension of its collaboration with the U.S. Food and Drug Administration (FDA). The 3DEXPERIENCE platform will be used to develop a new digital tool to enable more efficient regulatory review of cardiovascular and medical devices. Researchers hope the process will increase industry innovation and pave the way for an efficient path for patients to access safe, effective new treatments for the world’s leading cause of death – heart disease.
This second phase of their ongoing collaboration supports the 21st Century Cures Act, using virtual patients based on computational modeling and simulation to improve efficiency of clinical trials for new device designs. A groundbreaking project with the Living Heart simulated 3-D heart model will examine the use of heart simulation as a source of digital evidence for new cardiovascular device approvals. This includes an in silico clinical trial aimed to reduce animal testing or the number of patients required while still ensuring safety and efficacy of the device is demonstrated. The new digital process is intended to be more efficient and less expensive than current ones – whose delays and costs can impede patient access to novel treatments – without losing rigor or confidence in a device’s safety and efficacy.
The FDA has publicly recognized the public health benefits offered by modeling and simulation, and the potential for in silico clinical trials to safely advance medical products more efficiently, from preclinical studies through clinical trials to market.
“Modeling and simulation can help to inform clinical trial designs, support evidence of effectiveness, identify the most relevant patients to study and assess product safety. In some cases, in silico clinical trials have already been shown to produce similar results as human clinical trials,” said Tina Morrison, Ph.D., deputy director in the Division of Applied Mechanics, Office of Science and Engineering Labs, Center for Devices and Radiological Health, FDA. “The FDA continues to encourage research to facilitate the introduction of safe and effective therapeutic solutions.”
For more information: www.3ds.com

 May 12, 2020
May 12, 2020