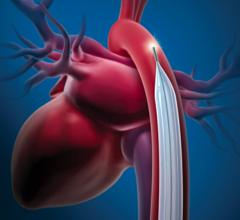
October 9, 2008 - Datascope Corp. said today it will sponsor a 300-patient, randomized clinical trial in high-risk acute myocardial infarction (MI) patients who are not in cardiogenic shock to show the efficacy of intra-aortic balloon counterpulsation in reducing infarct size.
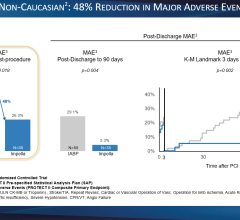
Patients enrolled in the CRISP AMI trial will be randomized equally to usual care versus usual care plus intra-aortic balloon counterpulsation (IABC) therapy prior to percutaneous coronary intervention. The primary efficacy endpoint will be a 25 percent reduction in infarct size due to IABC utilization. The successful completion of this trial represents a worldwide opportunity of more than 200,000 patients, or $200 million per year, the company said.
By targeting at-risk patients in this study, the aim is to significantly reduce infarct size, thereby improving myocardial salvage. Infarct size is a well-established surrogate clinical marker for patient mortality, congestive heart failure and quality of life.
The basis for the trial is a 2007 animal study. This study concluded that left ventricular unloading with IABC therapy prior to reperfusion reduces the extent of myocardial damage. These data suggest that in high-risk ST elevation myocardial infarction (STEMI) patients, IABP afterload reduction prior to reperfusion might be more beneficial than IABP placement post perfusion. This study was published in the October 2008 issue of Catheterization and Cardiovascular Interventions.
The trial will be conducted at approximately 30 sites in the U.S., Europe and Australia.
IABC therapy is currently the gold standard for a variety of clinical indications. It is a Class I indication for cardiogenic shock in the ACC/AHA guidelines.
For more information: www.datascope.com

 August 14, 2023
August 14, 2023