April 19, 2017 — Murj Inc., a digital health company that helps manage implantable cardiac device data, announced more than $4.5 million in financing. True Ventures led the most recent Series A financing. Social Capital joined the Series A, along with investors from Murj’s seed round.
Founded by former Apple product manager and Medtronic sales representative Todd Butka, Murj aims to liberate device clinics from paper processes and other ineffective device management tools.
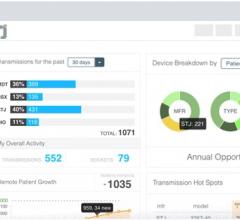
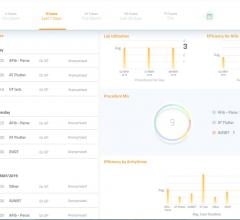
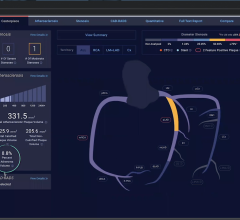
Today’s implantable cardiac devices such as pacemakers, loop recorders and implantable cardioverter-defibrillators (ICDs) continually generate important patient health and technical data. Current data management tools are inadequate, according to Murj, and clinicians are overwhelmed by the burden of managing device data, both remotely and when patients visit the office. The lack of effective tools makes it difficult for clinics to track patients and capture reimbursements, and an unexpected event such as a device recall can create an enormous paperwork burden.
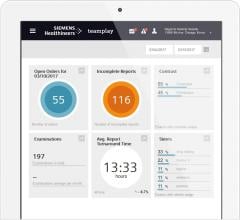
Murj is a cloud-based, HIPAA-compliant application accessed via a secure web browser. The application consolidates data from all implantable cardiac device types and manufacturers into a robust platform that delivers rapid clinical care and deep patient insight.
Murj will be on display at the Heart Rhythm Society (HRS) Scientific Sessions conference taking place May 10-13 in Chicago.
For more information: www.murj.com

 February 15, 2022
February 15, 2022