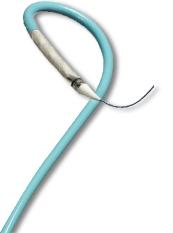
Image courtesy of Direct Flow Medical
January 19, 2015 — Direct Flow Medical Inc. announced the closing of a new round of financing for the company totaling $32 million. The company also announced the appointment of Chris Richardson as its first chief commercial officer to support global growth of the Direct Flow Medical Transcatheter Aortic Valve System. With the addition of Richardson, Dan Rose has been appointed vice president and general manager of Europe, the Middle East and Africa (EMEA).
The $32 million financing includes $17 million in private equity led by investor SV Life Sciences and the second tranche of a $50 million debt facility from PDL BioPharma worth $15 million. Proceeds will be used to complete the U.S. SALUS pivotal trial and fuel growth of the Direct Flow Medical system outside of the United States.
The benefits of the Direct Flow Medical Transcatheter Aortic Valve System are enabled by its design, which features a metal?free frame. Rather than a metal stent, the Direct Flow Medical System incorporates a polymer frame, which is expanded using pressurized saline and contrast for placement, assessment and repositioning. The saline/contrast solution is easily exchanged for a quick?curing polymer that solidifies and secures the valve in place once optimal positioning is reached.
The double?ring design of the valve creates a tight seal around the annulus. The system is fully repositionable and retrievable up until polymer exchange. The system avoids rapid pacing of the heart during deployment, and does not require post?dilatation following placement. The Direct Flow Medical Transcatheter Aortic Valve System is indicated for the treatment of patients with severe aortic stenosis who are at extreme risk for surgical aortic valve replacement (SAVR).The metal?free design enables a low?profile, fully sheathed delivery system for all valve sizes that minimizes vascular complications and improves hemodynamic outcomes.
The Direct Flow Medical Transcatheter Aortic Valve System is commercially available in Europe. In the United States, the company is working towards regulatory approval and is currently enrolling patients in its SALUS pivotal trial.
For more information: www.directflowmedical.com


 December 24, 2025
December 24, 2025 









