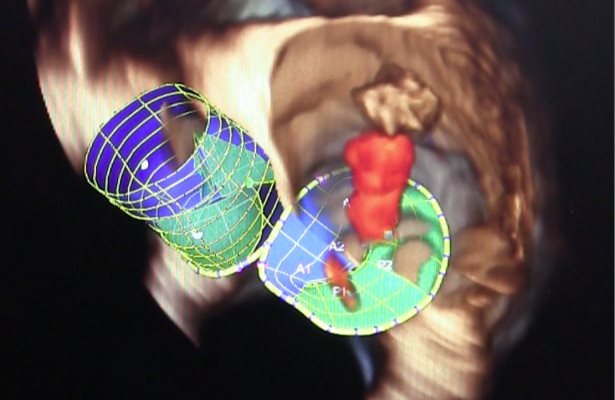
February 18, 2016 — Direct Flow Medical Inc. announced its transcatheter mitral valve (TMV) development program at the 2nd Annual Zurich Heart Team Mitral Valve meeting by featuring the Direct Flow Medical Transcatheter Mitral Valve in a preclinical case presentation.
The Direct Flow Medical Transcatheter Mitral Valve is built upon the conformable, repositionable and retrievable Direct Flow Medical Transcatheter Aortic Valve. The aortic valve has more than 2,500 implants and excellent published clinical results including low rates of paravalvular leak, pacemaker implant and mortality. The mitral-specific valve design features low atrial profile, low ventricular projection, and unique conformable sealing and fixation rings for the complex mitral annulus.
Azeem Latib, M.D., San Raffaele Hospital, Milan, presented preclinical results demonstrating transapical implant feasibility. Latib noted, “The valve’s unique conformable ring design is ideally suited for the complex shape of the mitral annulus.” He said, “Implanting this valve was similar to implanting the Direct Flow Medical Transcatheter Aortic Valve as I had total procedural control and performed a full hemodynamic assessment prior to deployment.”
Direct Flow Medical President and CEO Dan Lemaitre said that much of the mitral valve’s preclinical testing is completed, and the company hopes to achieve a first-in-man implant objective in the fourth quarter of 2016.
For more information: www.directflowmedical.com


 December 24, 2025
December 24, 2025 









