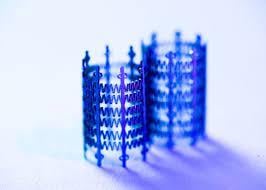
Draper engineers are developing the Low-force Expanding/Adaptable Pediatric (LEAP) Valve—the first heart valve that can grow with the patient. Credit: Draper
September 28, 2022 — Babies born with heart valve defects will have as many as 3 to 5 open heart surgeries before adulthood to replace prosthetics as they grow. Mortality and morbidity remain high, in part because there is no clinical standard for valve replacement in children under 5 years of age.
Eliminating even one of these surgeries would relieve these patients and their families of the health risk, stress and financial burden of a hospital stay and recovery. To address this need, Draper engineers are developing the Low-force Expanding/Adaptable Pediatric (LEAP) Valve—the first heart valve that can grow with the patient.
The LEAP Valve features a stent that can expand from 7 mm to 14 mm in diameter—making it ideal for infants through children up to 5 to 6 years old. As the patient grows, the device expands passively, applying a low outward force—much like a spring—eliminating the need for a balloon catheter or other invasive methods to mechanically expand the device.
“Draper is helping children, families and physicians through the development of the passively expanding LEAP Valve which would reduce the number of surgeries needed for 0-to-6-year-old children born with defects,” said Corin Williams, senior member of Draper’s Technical Staff and principal investigator of the LEAP heart valve.
Preclinical studies of the LEAP Valve are currently underway at Boston Children’s Hospital and Seattle Children’s Hospital.
Recently, Williams and her team developed an alternative to suturing the device to a bioprosthetic valve. The “sutureless” alternative, known as MANTIS (Mechanical Adhesion to Tissue), relies on microstructures for mechanical integration. Current practices require suturing by hand, which is both time-consuming and expensive when trying to construct smaller devices. MANTIS will allow faster and less labor-intensive device manufacturing.
In developing MANTIS, Draper turned for inspiration to its work on DARPA’s Z-Man program. For Z-Man, Draper developed a reversible adhesive elastomer material called MicroHold as part of a system that allows humans to climb glass walls.
Daniel King, senior member of Draper’s Technical Staff and a member of the LEAP Valve team, explains: “MANTIS builds upon principles of manufacturing microstructures that were first developed for the MicroHold material. The LEAP heart valve will incorporate the microstructures we are developing to integrate mechanically with the bioprosthetic valve tissue without the need for sutures.”
The MANTIS technology may have other applications, including hemorrhage control. “Additional development of MANTIS could open the door to a range of potential use cases, including external wound closure, sealing of internal organ lacerations and anastomosis, a procedure that involves connecting vessels and similar parts of the body,” King added.
Williams said the next major step for the LEAP Valve will be an Investigational Device Exemption submission to the FDA for first-in-human early feasibility studies. “Our goal is approval via the Humanitarian Device Exemption Pathway, with initial use in the lower risk pulmonary valve position, and eventual application to all 4 valve positions.”
Initially funded internally, the LEAP Valve won recognition for innovation and a prestigious FDA P50 grant from the Children’s National Health System. It was also recognized by the American Society for Artificial Internal Organs and the Pediatric Device Innovation Symposium. Recently, the LEAP Valve was named a 2022 Peer Reviewed Medical Research Highlights by the Department of Defense’s Congressionally Directed Medical Research Programs.
This work was supported by the U.S. Army Medical Research Acquisition Activity, through the program “Growth-Adaptive Pediatric Heart Valves: Addressing a Critical Unmet Need for Infants and Young Children That Saves Lives and Reduces Surgeries” under award number W81XWH2010295. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
For more information: http://www.draper.com


 November 24, 2025
November 24, 2025 









