March 27, 2023 — Patients with atrial fibrillation (AF) benefit from early rhythm control therapy. It reduces cardiovascular deaths, strokes, and other adverse outcomes by 20% compared to usual care. The beneficial effects of early rhythm control were shown by the pan-European EAST – AFNET 4 trial and confirmed by other large health studies. However, what is the price of the new treatment strategy? A cost-effectiveness analysis revealed: the health benefits of early rhythm control come at reasonable additional costs. The analysis was published in EP Europace, a journal of the European Society of Cardiology (ESC)1.
Atrial fibrillation is a rising epidemic. The number of AF patients in the European Union is projected to increase to approximately 18 million by 2060. Affected persons are at higher risk for stroke and other adverse events associated with high costs for treatment and long-term care. This leads to an increasing economic burden.
The EAST – AFNET 4 (Early Treatment of Atrial Fibrillation for Stroke Prevention) trial investigated whether rhythm control therapy – with antiarrhythmic drugs or atrial fibrillation ablation – delivered within one year after AF diagnosis improves outcomes. The main study result, published in 20202, demonstrated that early rhythm control therapy improves outcomes in patients with AF and comorbidities: early rhythm control with antiarrhythmic drugs and/or AF ablation reduced the primary outcome, a composite of cardiovascular death, stroke, and hospitalization for worsening heart failure or acute coronary syndrome, in 2,789 patients with early AF and cardiovascular risk factors compared to usual care over a 5-year follow-up.
“The health benefit of early rhythm control is evident. However, the cost-effectiveness of the new treatment strategy has not been evaluated so far. One concern after the publication of the EAST – AFNET 4 main study was whether the additional treatment would add an acceptable or undue financial burden to healthcare systems. In the current analysis, we examined for the first time the cost-effectiveness of early rhythm control compared to usual care,” explained Sophie Gottschalk, Department of Health Economics and Health Services Research, University Medical Center Hamburg-Eppendorf, Hamburg Center for Health Economics, Hamburg, Germany.
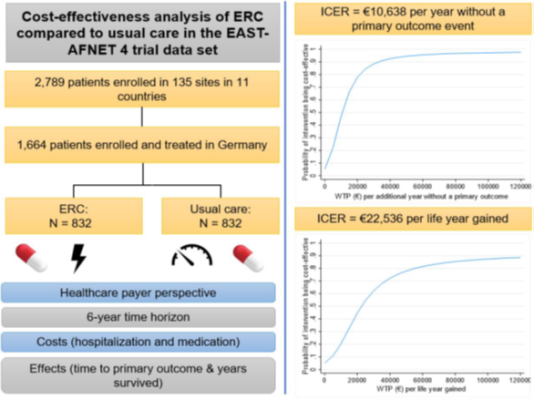
The cost-effectiveness analysis was based on data from the German subsample of the EAST – AFNET 4 trial comprising 1,664 patients (see graphical abstract). Early rhythm control in 832 patients was compared to usual care in 832 patients over a 6-year time horizon and from a German healthcare payer perspective. Cost categories considered in the analysis were hospitalization and medication costs. The time to the occurrence of a primary outcome and the time survived in the observation period were used as effect measures.
Incremental cost-effectiveness ratios (ICERs) were calculated for each effect measure. The ICER value describes the additional costs needed for an additional year free of primary outcome events or an additional life year, respectively. The analysis showed: early rhythm control was associated with higher costs to the amount of €1,924, resulting in ICERs of €10,638 per additional year without a primary outcome, and €22,536 per life year gained. As shown in the uncertainty analysis, high probabilities of cost-effectiveness could be reached if the healthcare payer was willing to pay ≥€55,000 for an additional year without a primary outcome or for a life year gained (probabilities ≥95% or ≥80%, respectively).
The principal investigator of EAST – AFNET 4, Prof. Paulus Kirchhof, Department of Cardiology, University Heart and Vascular Center Hamburg-Eppendorf, Hamburg, Germany, concluded: “The health benefits of early rhythm control come at reasonable additional costs as indicated by our analysis of the EAST – AFNET 4 study data for Germany. The added cost is comparable to the cost of other therapies reducing outcomes, and within typical ranges of acceptable payments in Germany. Future studies examining the cost-effectiveness of early rhythm control in other countries, in subgroups with higher benefit from rhythm control therapy, or the cost-effectiveness of different modes of early rhythm control are warranted.”
Since the publication of the main study result in 2020, different subgroup analyses of the EAST – AFNET 4 study data have been performed. One described the different, variable treatment patterns of antiarrhythmic drugs and AF ablation used in the trial, applied within guideline recommendations 3. Other subgroup analyses demonstrated the prognostic benefit of early rhythm control in patients with AF and heart failure4, in patients with asymptomatic AF5, in patients with different AF patterns6, in patients with high comorbidity burden7 and in patients with prior stroke8. A mediator analysis identified sinus rhythm as a key factor for the effectiveness of early rhythm control9.
The EAST – AFNET 4 trial has partners across Europe including the European Heart Rhythm Association (EHRA) of the ESC. For more information: www.af-net.eu
Notes: ERC, early rhythm control therapy; ICER, incremental cost-effectiveness ratio; primary outcome event = cardiovascular death, stroke, or hospitalisation for stroke or acute coronary syndrome.
References
1 Gottschalk S, Kany S, König H-H, Crijns HJGM, Vardas P, Camm AJ, Wegscheider K, Metzner A, Rillig A, Kirchhof P, Dams J. Cost-effectiveness of early rhythm control vs. usual care in atrial fibrillation care: an analysis based on data from the EAST-AFNET 4 trial. EP Europace. 2023. doi:10.1093/europace/euad051.
https://academic.oup.com/europace/article-lookup/doi/10.1093/europace/euad051
2 Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, Hamann F, Heidbüchel H, Hindricks G, Kautzner J, Kuck K-H, Mont L, Ng GA, Rekosz J, Schön N, Schotten U, Suling A, Taggeselle J, Themistoclakis S, Vettorazzi E, Vardas P, Wegscheider K, Willems S, Crijns HJGM, Breithardt G, for the EAST–AFNET 4 trial investigators. Early rhythm control therapy in patients with atrial fibrillation. N Engl J Med. 2020; 383:1305-1316.
DOI: 10.1056/NEJMoa2019422
3 Metzner A, Suling A, Brandes A, Breithardt G, Camm AJ, Crijns HJGM, Eckardt L, Elvan A, Goette A, Haegeli LM, Heidbuchel H, Kautzner J, Kuck KH, Mont L, Ng GA, Szumowski L, Themistoclakis S, van Gelder IC, Vardas P, Wegscheider K, Willems S, Kirchhof P. Anticoagulation, therapy of concomitant conditions, and early rhythm control therapy: a detailed analysis of treatment patterns in the EAST - AFNET 4 trial. EP Europace. 2022; 24:552–564. DOI: 10.1093/europace/euab200
4 Rillig A, Magnussen C, Ozga, Suling A, Brandes A, Breithardt G, Camm AJ, Crijns HJGM, Eckardt L, Elvan A, Goette A, Gulizia M, Haegeli LM, Heidbuchel H, Kuck KH, Ng GA, Szumowski L, van Gelder IC, Wegscheider K, Kirchhof P. Early rhythm control therapy in patients with heart failure. Circulation. 2021;144(11):845-858. DOI: 10.1161/CIRCULATIONAHA.121.056323
5 Willems S, Borof K, Brandes A, Breithardt G, Camm AJ, Crijns HJGM, Eckardt L, Gessler N, Goette A, Haegeli LM, Heidbuchel H, Kautzner J, Ng GA, Schnabel R, Suling A, Szumowski L, Themistoclakis S, Vardas P, van Gelder IC, Wegscheider K, Kirchhof P. Systematic, early rhythm control therapy equally improves outcomes in asymptomatic and symptomatic patients with atrial fibrillation: the EAST-AFNET 4 Trial. Eur Heart J. 2022; 43:1219-1230. DOI: 10.1093/eurheartj/ehab593.
6 Goette a, Borof K, Breithardt G, Camm AJ, Crijns H, Kuck KH, Wegscheider K, Kirchhof P, MD. Presenting Pattern of Atrial Fibrillation and Outcomes of Early Rhythm Control Therapy. J Am Coll Cardiol. 2022; 80:283-95. DOI: 10.1016/j.jacc.2022.04.058
7 Rillig A, Borof K, Breithardt G, Camm AJ, Crijns HJGM, Goette A, Kuck KH, Metzner A, Vardas P, Vettorazzi E, Wegscheider K, Zapf A, Kirchhof P. Early rhythm control in patients with atrial fibrillation and high comorbidity burden. Circulation. 15 Aug 2022. DOI:10.1161/CIRCULATIONAHA.122.060274
8 Jensen M, Suling A, Metzner A, Schnabel R, Borof K, Goette A, Haeusler KG, Zapf A, Wegscheider K, Fabritz L, Diener H-C, Thomalla G, Kirchhof P. Early rhythm-control therapy for atrial fibrillation in patients with a history of stroke: a subgroup analysis of the EAST- AFNET 4 trial. Lancet Neurol, 2023; 22: 45–54. DOI: 10.1016/PIIS1474-4422(22)00436-7
9 Eckardt L, Sehner S, Suling A, Borof K, Breithardt G, Crijns HJGM, Goette A, Wegscheider K, Zapf A, Camm AJ, Metzner A, Kirchhof P. Attaining sinus rhythm mediates improved outcome with early rhythm control therapy of atrial fibrillation: the EAST – AFNET 4 trial. Eur Heart J, 2022. DOI: 10.1093/eurheartj/ehac471


 August 29, 2025
August 29, 2025