May 15, 2012 — A simple clinical assessment performed in the doctor’s office can identify patients who are at high risk for stroke and other major complications following a procedure to clear blockages from the neck arteries that supply blood to the brain, according to a study presented at the SCAI 2012 Scientific Sessions.
The assessment, which includes just five clinical characteristics and requires no invasive tests, provides valuable guidance to patients considering stenting for carotid artery disease.
“We can see someone in the office and gauge risk very quantitatively without having to do angiography,” said Beau Hawkins, M.D., an interventional cardiology fellow at Massachusetts General Hospital in Boston. “That gives patients important information. It allows them to be more active in the decision-making process.”
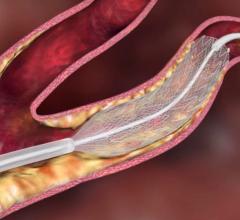
Carotid artery stenting involves threading a slender tube (catheter) into the carotid arteries in the neck, inflating a tiny balloon to widen the artery and push aside cholesterol plaque, and placing an expandable metal mesh stent to prop open the artery. Stenting is less invasive alternative to surgery in some patients with neck blockages, especially those who are at high risk for surgical complications.
For the study, researchers analyzed data from thousands of patients in the National Cardiovascular Data Registry (NCDR) CARE Registry, which collects clinical information and procedural results on patients treated with carotid artery stenting. Among the 11,122 procedures the researchers reviewed, there were a total of 304 major complications such as stroke, heart attack or death (2.7 percent). After sifting through more than 30 characteristics from the patients’ clinical histories and x-ray angiography tests, Dr. Hawkins and his colleagues developed a risk score based on five factors that significantly increased the likelihood of major complications after carotid artery stenting.
These factors and their contribution to the risk score are outlined below:
- Age (plus 2 points for each decade over age 50, up to a maximum of 10 points)
- Atrial fibrillation (plus 2 points)
- Previous stroke (plus 3 points)
- Stroke-like symptoms from the carotid artery blockage within the previous six months (plus 2 points)
- Major surgery planned in the near future (plus 4 points)
The researchers found that by using the risk score, they could predict the likelihood of major complications for a given patient. When patients were divided into low-, intermediate-, and high-risk groups by their risk score, the rates of major complications rose accordingly (1.4 percent, 4.0 percent and 7.1 percent, respectively).
“This gives both clinicians and patients a simple, easy-to-use tool that informs us about the patient’s risk of undergoing carotid artery stenting,” Hawkins said. “That’s important—and much needed—because it tells us who is at exceptionally high risk and probably should consider other treatments for their carotid artery disease.”
This research was supported by the National Cardiovascular Database Registry.
Hawkins reports no potential conflicts of interest.
For more information: www.scai.org

 June 26, 2023
June 26, 2023