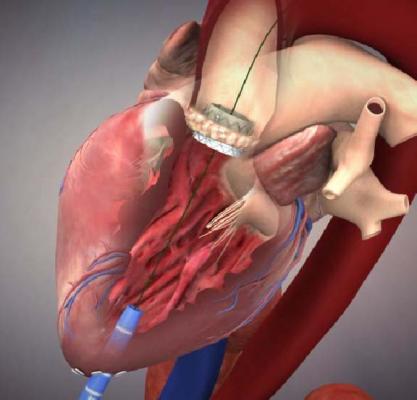
Edwards Receives IDE for U.S. Clinical Trial of Sapien 3 Transcatheter Valve
August 6, 2013 — Edwards Lifesciences received conditional investigational device exemption (IDE) approval from the U.S. Food and Drug Administration (FDA) to initiate a clinical trial of its Edwards Sapien 3 transcatheter aortic heart valve and accessories.
The trial will study the Sapien 3 valve in the treatment of high-risk and inoperable patients with severe symptomatic aortic stenosis. It will enroll up to 500 patients treated with one of three delivery techniques: the transfemoral approach through an incision in the leg, the transapical route between the ribs, or the transaortic approach through a small incision in the chest and aorta. Edwards anticipates the trial will have a one-year composite endpoint compared to previous Sapien valves.
"We're very excited to have the opportunity to initiate a U.S. study of the Sapien 3 valve. This valve builds on the Sapien platform and provides new features designed to benefit patients and enhance ease of use, such as a lower profile, a fabric cuff intended to reduce paravalvular leak and new delivery systems," said Larry L. Wood, corporate vice president, transcatheter heart valves. "We appreciate the FDA's efforts to allow initiation of this trial in a timely way, providing U.S. patients with access to our most advanced transcatheter system."
The Edwards Sapien 3 valve is an investigational device and is not available commercially in any country.
For more information: www.edwards.com


 December 24, 2025
December 24, 2025 









