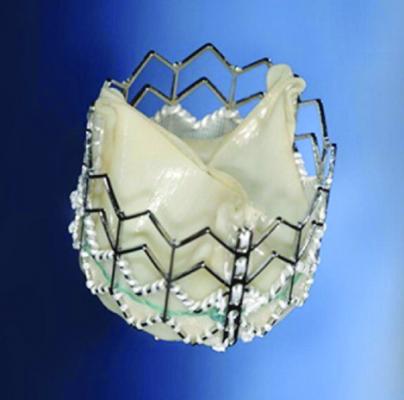
August 27, 2013 — Edwards Lifesciences Corp. announced that Japan's Central Social Insurance Medical Council (Chuikyo) has approved the recommendation by the Japanese Ministry of Health, Labor and Welfare's (MHLW) expert review panel to provide reimbursement for the Edwards Sapien XT transcatheter aortic heart valve. The reimbursement is scheduled to go into effect on Oct. 1.
This decision follows regulatory approval of the Sapien XT valve in June. With this approval, Chuikyo established a reimbursement of 4.53 million yen (approximately $46,000 at current exchange rates) for when the Sapien XT valve is used in the treatment of patients with severe symptomatic aortic stenosis. The reimbursement rate for medical devices in Japan covers the cost of the device as well as taxes, certain hospital fees and distribution expenses.
The Sapien XT valve is the first commercially available transcatheter valve in Japan. In the United States, the Sapien XT valve is not commercially available; it is an investigational device being studied as part of a randomized, pivotal clinical trial.
For more information: www.edwards.com


 December 24, 2025
December 24, 2025 









