October 3, 2023 — EnCompass Technologies, Inc., a privately held medical device company focused on protecting patients from brain injury during cardiovascular procedures, announced the US Food and Drug Administration (FDA) officially granted conditional Investigational Device Exemption (IDE) approval for its F2 cerebral embolic protection system on Friday, September 22, 2023.
While interventional therapies for structural heart disease have significantly improved the quality of life and longevity of many patients, brain injury (including stroke) remains a feared complication. All cardiovascular procedures cause the release of particulate debris (calcium, vessel wall, blood clot) and air bubbles, called emboli. The emboli can cause injury if they reach the brain, affecting one person about every 30 seconds globally.1 The severity of brain injury typically depends on the size and number of emboli. Such emboli often are small and most are less than 40 micrometers (µm) in size.2
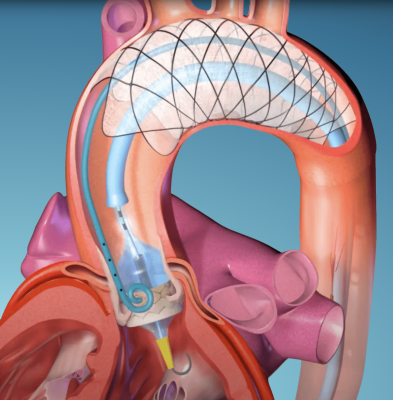
The F2 filter by EnCompass is currently the only filter with pores small enough to block most emboli to the brain while preserving blood flow. The F2 filter has the smallest average pore size among all CEP devices at 30 µm. During the TAVR procedure, 360-degree wall apposition of the F2 filter in the aortic arch is designed to prevent migration. Because the F2 filter is attached to a self-expanding nitinol stent, it is easy to insert, deploy, and retrieve.
At the 21st Annual Meeting of the Society of Neurointerventional Surgery (August 14-17, 2023), researchers from UCLA presented an in vitro study comparing the F2 filter versus Boston Scientific's Sentinel™. Using a 3D printed silicone model of the aortic arch perfused with saline at 5 L-min-1, artificial emboli of three sizes (45-53 µm, 106-125 µm, and 250-300 µm) were injected into the aortic root. Effluent was continuously collected from the left and right common carotid and vertebral arteries. For all sizes of emboli, the F2 filter prevented 94 percent more emboli from reaching the brain than Sentinel™.3 In April 2023, a first-in-human (FIH) study began in Tbilisi, Georgia, where three patients undergoing TAVR received the F2 filter. This small cohort of patients demonstrated 100 percent procedural success and no adverse events. In September 2023, a similar pilot study began in Australia and now with FDA conditional IDE approval, EnCompass plans to commence its first US pilot study. These pilot clinical studies will lead to a US pivotal clinical trial in 2024.
"Brain injury after TAVR, as well as other cardiovascular procedures, remains a significant problem. As most of these complications are caused by emboli, it makes sense to use a filter that blocks most harmful emboli from entering the brain. The US pilot study, along with our growing clinical experience outside the US, represents the beginning of our clinical journey towards demonstrating meaningful patient benefits," said George Wallace, CEO of EnCompass Technologies.
For more information: www.encompassf2.com
References:
1. Wolman, R. L., Nussmeier, N. A., Aggarwal, A., et al. Cerebral injury after Cardiac Surgery. Stroke, 30(3), 514–522. https://doi.org/10.1161/01.str.30.3.514
2. Pugsley et al. Stroke 1994;25:1393-1399.
3. https://jnis.bmj.com/content/15/Suppl_1/A128.abstract


 January 15, 2026
January 15, 2026 









