February 12, 2009 - Endologix Inc. yesterday said a live-case demonstration of its new IntuiTrak Delivery System was featured at the International Congress XXII Endovascular Interventions conference in Scottsdale, AZ.
The procedure was performed by Zvonimir Krajcer, M.D., co-director of the peripheral vascular disease service at the Texas Heart Institute; Edward Diethrich, M.D., medical director and founder of the Arizona Heart Institute; and Venkatesh G. Ramaiah, M.D., vascular surgeon at the Arizona Heart Hospital. The endovascular AAA procedure was successfully performed with a percutaneous approach and local anesthesia.
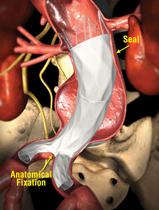
IntuiTrak was developed for the minimally invasive delivery and deployment of the Powerlink stent graft. The device is currently in limited market release following FDA approval in October 2008. The company said IntuiTrak’s design and deployment mechanism simplifies standard vascular delivery of the Powerlink device and provides exceptional accuracy and control. The low-profile delivery system features enhanced flexibility, advanced hemostasis control and a hydrophilic coating to facilitate smooth delivery, particularly in patients with limited or difficult vascular access, the company said. Additionally, the delivery catheter has an integrated sheath that minimizes the need to exchange introducers, thereby having the potential to minimize vessel trauma and reduce overall procedure time.
“Due to its low-profile and integrated sheath, IntuiTrak is ideal for the percutaneous treatment of patients with AAA,” said Dr. Krajcer. “I have used the new device in eight cases so far, and have experienced 100 percent technical success and exceptional patient outcomes.”
For more information: www.endovascularcongress.org, www.endologix.com


 October 28, 2025
October 28, 2025 









