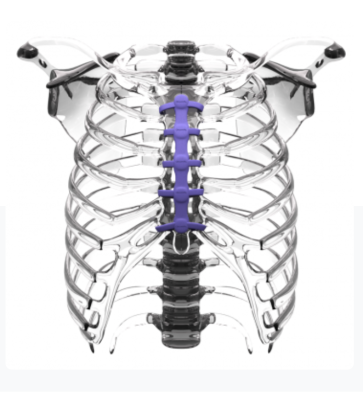
CircumFix’s medical device is composed of a slender chest plate placed on the sternum and held in place by fasteners that encircle the sternum and are affixed to the plate.
January 18, 2022 – Evonik Venture Capital has invested in CircumFix Solutions, a Tennessee-based start-up that has developed a new sternal closure device to improve patient recovery after open chest surgery. The patented orthopedic device, made of a high-performance polymer from Evonik, closes and holds the sternum securely together after surgery.
“We are seeing a revolution in implantable medical devices with the shift from metal to high-performance polymers and this investment supports that shift,” said Bernhard Mohr, head of Evonik Venture Capital. “Patients and doctors can benefit enormously from a technology that is safe, reliable and enhances recovery.”
CircumFix’s medical device is composed of a slender chest plate placed on the sternum and held in place by fasteners that encircle the sternum and are affixed to the plate. The construct allows for even “load sharing” of bone and device and restricts motion, which increases stability and reduces pain. Surgeons can close the sternum faster and more easily than with traditional devices. Should a further operation be needed the device can be quickly reopened and closed again. It is safe and comfortable as a permanent implant. In comparison to some metal devices currently on the market, bones and tissue aren’t damaged.
The Evonik material used in the closure device is an implant grade polyether ether ketone, or PEEK. The material is biocompatible - not harmful or toxic to living tissue – and bioinert – it doesn’t initiate a response from the body. These properties as well as being hydrophobic – repelling water – significantly reduce the chance of infection. Being made from PEEK polymer means that the device is transparent to x-rays and therefore doesn’t interfere with post-surgical diagnostics.
“The investment will deepen our relationships across the medical technology industry and with engineering companies,” said Marc Knebel, head of Medical Systems at Evonik. “At the same time, we can reach U.S. clinical experts in orthopedics, many of whom are less aware of PEEK as an alternative to titanium and stainless steel.”
The U.S. sternal closure device market was estimated at $1.4 billion for 2021 with more than 700,000 procedures performed per year. The market is expected to grow at a compound annual growth rate of 5 percent reaching $1.9 billion by 2026.
The investment will allow Evonik to build on expertise already acquired in implant-grade high-performance polymers. PEEK is already used in spine, skull, jaw and face surgery as well as orthopedics and has high potential to be used in other medical applications.
Circumfix Solution, Inc. founders Louis Houff and Ken Richardson have more than 50 years combined experience in orthopedic sales, marketing, product development and senior leadership roles. They founded the company in 2020 recognizing the need for improved sternum closure techniques and materials. Houff first conceived of the novel bone fixation and healing concept in 2012 and received his first patent in 2017.
“We are making great progress towards the goal of improving patient recovery,” said Houff, who is also chief executive officer of CircumFix. “With support from Evonik, we will be able to further drive business development forward.”
Evonik markets its high-performance polymer Polyether ether ketone under the name VESTAKEEP.
For more information: https://circumfix.com/


 January 15, 2026
January 15, 2026 









