March 16, 2010 – Preliminary results from the single-center study suggest that compared to SPECT myocardial perfusion imaging (MPI), flurpiridaz F18 PET MPI has a higher diagnostic specificity for detecting right coronary artery disease and a higher accuracy for evaluating the extent of stress-induced myocardial perfusion abnormalities with coronary angiography as the gold standard.
The data were featured today in a presentation by Jamshid Maddahi, M.D. FACC, professor of molecular and medical pharmacology (nuclear medicine) and medicine (cardiology) at the David Geffen School of Medicine at UCLA, and the lead principal investigator of the study, at the American College of Cardiology 59th Annual Scientific Session in Atlanta.
The results came from data on flurpiridaz F18 (formerly known as BMS747158), its novel compound in development as a positron emission tomography (PET) MPI agent.
The study of nine patients compared rest-stress flurpiridaz F18 PET MPI, with rest-stress technetium-99m (Tc-99m) labeled single photon emission computed tomography (SPECT) for the detection and evaluation of coronary artery disease (CAD).
“These initial results, in a very small patient population, are encouraging, as they already show significant improvements in diagnostic accuracy using PET imaging with flurpiridaz F18 as compared to SPECT imaging with Tc-99m for the detection and evaluation of coronary artery disease,” said Dr. Maddahi. “A product that could demonstrate increased diagnostic specificity would provide physicians with a useful tool to reduce the number of false positive results often associated with SPECT imaging, and, therefore reduce the number of unnecessary cardiac catheterizations.”
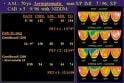
Nine patients from a single study center underwent same day rest-stress Tc-99m labeled SPECT MPI and separate day rest-stress flurpiridaz F18 PET MPI and coronary angiography. For each patient, 17 myocardial segments were visually scored for each rest and stress image by independent observers who were blinded to the other results. Summed stress scores, summed rest scores and summed difference scores were determined for each patient from the segmental scores. The percentage of narrowing in each coronary artery was evaluated blindly, and greater than 70 percent luminal diameter narrowing was considered significant.
Findings showed that the overall accuracy for correct identification of diseased coronary arteries was 93 percent (25/27) for PET and 78 percent (21/27) for SPECT. Six patients were found to have CAD with both SPECT and PET, and for the three patients without CAD, all three were found to be normal through PET imaging and two were found to be normal through SPECT. Of the nine diseased coronary arteries, PET detected all and SPECT detected eight. Of the 18 normal coronary arteries, PET imaging identified 16 of the 18 arteries while SPECT found 13 of the 18. Specificity for the right coronary artery was significantly higher through PET imaging compared to SPECT: 100 percent compared to 43 percent.
Flurpiridaz F18, a fluorine 18-labeled agent that binds to mitochondrial complex 1 (MC-1), was designed to be a novel myocardial perfusion PET imaging agent for the diagnosis of coronary artery disease (CAD). The agent is currently in phase two clinical trials. Phase one studies, also led by Dr. Maddahi, indicated that flurpiridaz F18 is well-tolerated and demonstrates radiation dosimetry that is comparable to, or less than, that of other PET imaging agents. The data also showed high myocardial uptake at rest that significantly increased with pharmacologically induced stress as well as a ratio of myocardial to background uptake that was favorable and improved over time, suggesting strong potential as a myocardial perfusion PET imaging agent for patients both at rest and under stress.
For more information: www.lantheus.com


 March 25, 2025
March 25, 2025 









