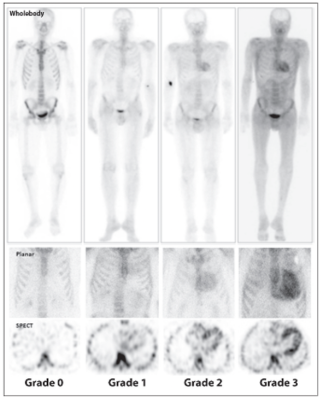
Examples of Grade 0–3 images. Visual grading scale for cardiac uptake on whole-body (top) and chest (middle) planar imaging, and chest SPECT (bottom) imaging (from left to right: grade 0, 1, 2, and 3).
June 24, 2022 — Shortages of pyrophosphate (PYP), the radiopharmaceutical most commonly used in the U.S. for noninvasive diagnosis of transthyretin (ATTR) cardiac amyloidosis, should not delay imaging in at-risk patients, according to a new information statement issued by the American Society of Nuclear Cardiology (ASNC). Instead, ASNC is guiding imagers to use 99mtechnetium (99mTc)-hydroxymethylene diphosphonate (HMDP or HDP) to ensure patients continue receiving prompt evaluation of, and treatment for, cardiac amyloidosis.
ASNC’s statement, titled “Radiopharmaceutical Supply Disruptions and the Use of 99mTc-Hydroxymethylene Diphosphonate as an Alternative to 99mTc-Pyrophosphate for the Diagnosis of Transthyretin Cardiac Amyloidosis,” is available online on the Zenodo open-data repository and is in-press with the Journal of Nuclear Cardiology.
Cardiologists’ understanding of cardiac amyloidosis has been transformed over the past decade as research revealed the condition is much more prevalent than previously believed and that patients can be accurately diagnosed without invasive cardiac biopsies.
A crucial component of diagnosing ATTR cardiac amyloidosis noninvasively is nuclear cardiology imaging using any of three bone-avid 99mtechnetium-labeled radiopharmaceuticals: 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid (DPD), 99mTc-pyrophosphate (PYP) or 99mTc-hydroxymethylene diphosphonate (HMDP). Of these three agents, only 99mTc-PYP and 99mTc-HMDP are approved for use in the U.S. by the FDA, and until recently, 99mTc-PYP has been virtually the only radiopharmaceutical used in the U.S. for the evaluation of suspected cardiac amyloidosis.
Since early 2022, nuclear cardiology laboratories have been grappling with 99mTc-PYP shortages caused by COVID-19-related supply chain disruptions. According to suppliers of radiopharmaceutical agents, the disruptions are expected to continue.
“With the recent supply disruptions in the availability of PYP and the importance of early diagnosis and prompt treatment of ATTR cardiac amyloidosis, we are encouraging physicians to use HMDP as an alternative imaging agent,” says Edward J. Miller, MD, PhD, FASNC, lead author of the ASNC information statement and vice chief of Cardiovascular Medicine and director of Nuclear Cardiology at the Yale School of Medicine. “Evidence suggests HMDP appears similar to PYP in its accuracy and image quality. ASNC’s new information statement will provide physicians with the information they need to leverage HMDP for their patients.”
ASNC has endorsed 99mTc-HMDP as a “reasonable alternative” to 99mTc-PYP because it delivers comparable image quality and diagnostic performance and because it has been used extensively internationally. The information statement explains –
The nomenclature and chemical structures of the relevant radiopharmaceuticals, including why HMDP (also abbreviated as HDP) must not be confused with methylene diphosphonate (MDP); How cardiovascular imaging professionals should alter image acquisition protocols and study interpretation when using 99mTc-HMDP vs. 99mTc-PYP; and The billing codes to use for reimbursement of HMDP studies in the U.S.
The statement also calls for more research comparing the bone-avid radiopharmaceutical agents in the context of amyloidosis diagnosis. ASNC sponsors an annual research grant competition where one early-career researcher receives funding to perform a mentored research project exploring the role of nuclear cardiology in the early detection of ATTR cardiac amyloidosis. The deadline to enter this year’s competition is July 5.
For more information: www.asnc.org


 January 05, 2023
January 05, 2023