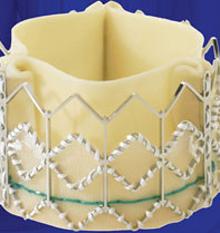
June 10, 2011 - A U.S. Food and Drug Administration (FDA) advisory panel is scheduled to review Edwards Lifesciences’ premarket approval (PMA) application for the Sapien transcatheter heart valve on July 20. Edwards submitted a PMA application in the fall of 2010 based on data from the inoperable cohort (Cohort B) of the PARTNER Trial, for approval of this therapy in the treatment of inoperable patients with severe aortic stenosis.
The inoperable cohort compared outcomes after treatment with either standard therapy or the Edwards Sapien valve in 358 patients. The results of this study were published in The New England Journal of Medicine in September 2010.
The Sapien heart valve is an investigational device and not yet available commercially in the United States.
If cleared for commercial use, it would become the first transcatheter aortic valve replacement device in the United States. Transcatheter valve replacement is expected to eventually replace the current standard of open-heart valve replacement surgery.
So far, only one transcatheter valve has been cleared by the FDA – the Medtronic Melody pulmonary valve, which was cleared under a humanitarian device exemption in early 2010.
Medtronic also entered U.S trials for its version of a transcatheter aortic valve – CorValve – in late 2010.
For more information: www.edwards.com


 December 24, 2025
December 24, 2025 









