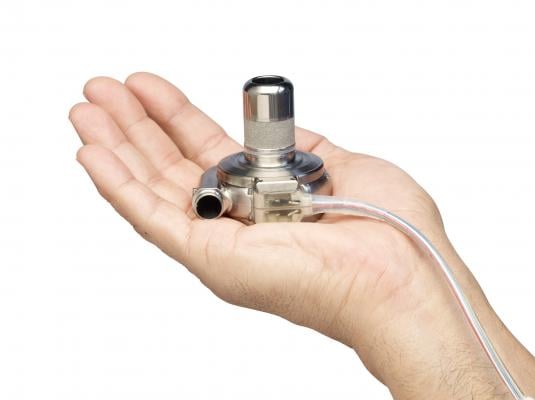
October 5, 2016 — Medtronic said two previously communicated global voluntary recalls related to the HeartWare International (HeartWare) HVAD System have been classified as Class 1 by the U.S. Food and Drug Administration (FDA). Class 1 recalls describe situations where there is reasonable risk of serious adverse health consequences or death.
In a safety notification letter distributed globally in May and June 2016, HeartWare notified physicians regarding potential damage to controllers from exposure to moisture through loose power and data connectors. In the U.S., all clinician notifications have been acknowledged, and globally 99 percent of clinician notifications have been acknowledged.
Hospital clinicians were advised to inspect patients' HVAD HeartWare Controllers for loose connectors at patients' regularly scheduled appointments and to replace affected controllers with a new controller at the clinicians' discretion. Clinicians also were advised to remind patients about the safe use of the HVAD System, particularly with regard to moisture and proper connection to power and data sources. Damage to the controllers from this issue could cause loss of communication between the controller and monitor, reduced ability to detect alarms or interruption of circulatory support due to pump stop, which could lead to serious injury or death.
HeartWare controllers subject to this safety notification include the following models sold worldwide: Model No. 1400 and 1401.
At the initiation of this recall, approximately 8,799 potentially affected HVAD HeartWare Controllers with these model numbers had been distributed and remained in use by patients, worldwide. As of Sept. 26, 2016, this recall and subsequent inspection of patients' controllers has resulted in the replacement of 308 affected HVAD controllers worldwide.
In August 2016, HeartWare issued a global voluntary recall of certain models of unimplanted, sterile HVAD Pump Implant Kits (pumps) in hospital inventory. The HVAD pumps contained in these sterile implant kits may be susceptible to electrical faults and connection failures if fluid enters the driveline-to-controller connector during or after the implant procedure. Electrical faults or connection failures could interrupt circulatory support due to a pump stop, potentially resulting in serious injury or death. In the U.S., all clinician notifications have been acknowledged, and globally 89 percent of clinician notifications have been acknowledged.
Clinicians were advised to review hospital inventories for HVAD implant kits (pumps) with serial numbers lower than HW25838 with the following model number and notify the company for replacement: Model No. 1103 and 1104.
At the initiation of this recall, 350 potentially affected HeartWare HVAD implant kits with these model numbers had been distributed and remained in hospital inventories, worldwide. As of Sept. 26, 2016, 323 of the 350 implant kits, or 92 percent, have been used or returned to HeartWare.
Medtronic acquired HeartWare on Aug. 23, 2016. The combined organization is committed to putting patient safety and customers first and to implementing manufacturing enhancements to address these issues.
The HVAD System includes a ventricular assist device (VAD), or mechanical pump, that pumps blood to the body when one of the heart's natural pumps (a ventricle) does not perform well. The controller is a small computer that monitors the pump. These implanted systems can allow people with advanced heart failure to return to a fuller life while they await heart transplantation.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online at www.fda.gov/medwatch/report.htm.
For more information: www.medtronic.com


 January 15, 2026
January 15, 2026