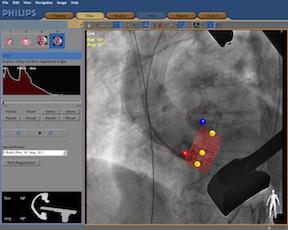
December 23, 2011 - Philips Healthcare said Medical City Dallas Hospital, Dallas, Texas, is the first hospital in the United States to use Philips’ HeartNavigator interventional tool in clinical practice. HeartNavigator is a new procedure planning and image guidance tool to help interventional cardiologists and cardiac surgeons perform minimally invasive heart valve replacements. Developed in cooperation with partner hospitals around the world, HeartNavigator is designed to increase the objectivity of the procedure planning and to simplify the procedure itself.
HeartNavigator was commercially introduced in Europe in the first quarter of 2011 and recently received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
“After the recent commercial introduction of transcatheter heart valves in the U.S., we are now able to offer heart valve implantations to a group of patients for whom the risks associated with open heart surgery are too high,” said Todd M. Dewey, M.D., cardiothoracic surgeon at Medical City Dallas Hospital. “The implantation of a heart valve via a catheter has the advantage of being minimally invasive, but it demands high-quality imaging and precise navigation to ensure accurate positioning. HeartNavigator gives us a much better view of the procedure, which aids us in the preparation of the procedure as well as the execution.”
In contrast to open heart surgery, which involves significant opening up of the patient’s chest, minimally invasive heart valve replacement only requires a small incision, through which catheters are inserted and guided to the heart with the aid of dedicated interventional X-ray systems. Because the artificial valves required for such procedures were only recently commercially introduced in the United States., the adoption of minimally invasive heart valve replacement in the U.S. is still in its early stages, but is expected to increase rapidly. In Europe, where such valves have been commercially available since 2008, it is estimated that more than 13,000 minimally invasive valve replacements were performed in 2010 – a number that has been growing at high double-digit growth rates year-on-year.
“I plan all my cases with the HeartNavigator. I trust the measurements with HeartNavigator more than the measurements provided by the normal CT scan,” said H. Schröfel, M.D., Senior Cardiac Surgeon at the Karlsruhe Heart Surgery Clinic, Germany.
To date a total of 895 transcatheter aortic heart valve replacement procedures have been performed at the Karlsruhe Heart Surgery Clinic.
Because minimally invasive heart valve replacement procedures deprive physicians of the ability to directly see and touch the patient’s heart, they are complex and technically demanding. Philips has developed a new technology that merges pre-operatively acquired 3-D CT scans of the patient’s heart with the live interventional X-ray views. Using this technology, physicians can now simultaneously see the detailed 3-D anatomy of the patient’s heart together with the positioning of the catheter and the placement and deployment of the artificial valve.
For more information: www.philips.com


 December 24, 2025
December 24, 2025 









