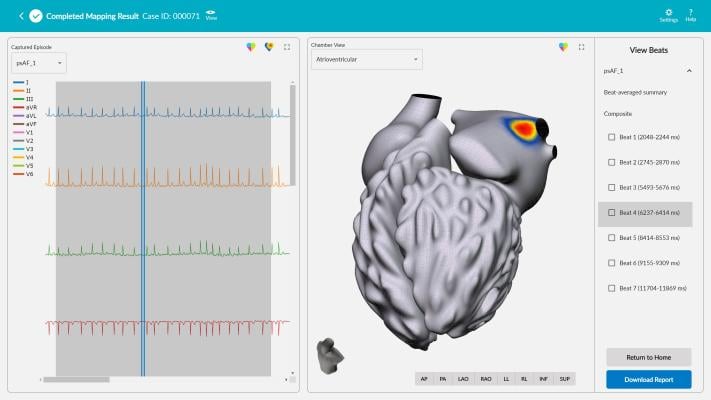
November 15, 2021 — Vektor Medical Inc. announced U.S. Food and Drug Administration (FDA) 510(k) clearance for its computational ECG mapping system, vMap. vMap is designed to map potential arrhythmia sources (hot spots) associated with stable or unstable arrhythmias anywhere in the heart—including all four chambers, the septal wall, and the outflow tracts—in less than three minutes using only ECG data, to improve outcomes in ablation procedures. Following clearance, the vMap system will be commercially available to sites across the U.S.
vMap has demonstrated success in identifying arrhythmia sources for a wide variety of arrhythmias, including atrial fibrillation. The easy-to-use system takes less than three minutes for a clinician to input case information, download and markup an ECG in the system, and receive a three-dimensional, interactive arrhythmia hot spot map visualizing the inside and outside of the heart. vMap can be used as a non-invasive standalone tool or as a complement to traditional invasive electro-anatomical mapping systems in planning and procedural settings.
“Traditional arrhythmia mapping techniques are labor-and time-intensive, and physicians are only able to achieve complete success in a limited number of ablation procedures due to the lack of information on arrhythmia source locations. To shorten procedure times and improve ablation success, electrophysiologists need to be able to visualize arrhythmia hot spots in the heart quickly and accurately,” said Amir Schricker, M.D., MS, medical director of cardiac electrophysiology at Mills Peninsula Medical Center, a Sutter Health hospital. “Our clinical experience with vMap has been incredibly positive. Using non-invasive ECG data, the system is extremely simple and fast to use, and quickly provides a hot spot map so we know where to target our efforts without having to navigate the whole heart or order additional imaging.”
“Cardiac arrhythmias impact millions of people across the globe, increasing the risk of serious health-related issues, such as stroke, heart failure, and even death. Yet, today’s therapies have significant issues - drug therapy can have severe side effects and non-targeted ablation has disappointingly low success rates,” said Vektor Medical CEO Mike Monko. “With vMap we are changing how electrophysiologists think about mapping. By providing a hot spot map in only minutes based on non-invasive ECG data, physicians can create a more effective ablation plan and spend less time finding target locations. Our goal is to increase first-pass success rates, lower risk, and decrease the current cost burden of ablation on the healthcare system.”
San Diego-based Vektor Medical Inc. is the developer of the world’s first technology to rapidly map arrhythmias anywhere in the heart using only 12-lead ECG data. This data is analyzed using proprietary computational modeling to create actionable 2D and 3D maps of potential arrhythmia sources. The company’s smart, simple, and non-invasive cardiac arrhythmia mapping platform aims to improve first-pass ablation success, lower risks from invasive mapping and long fluoro exposure, and reduce procedure times1, all of which are expected to reduce healthcare costs associated with ablation.
For more information: www.vektormedical.com
Reference:
1 Ho, G. et al. Simplified workflow to improve the precision of non-invasive radio-ablation of ventricular tachycardia storm. Presented at AHA 2020.


 January 15, 2026
January 15, 2026 









