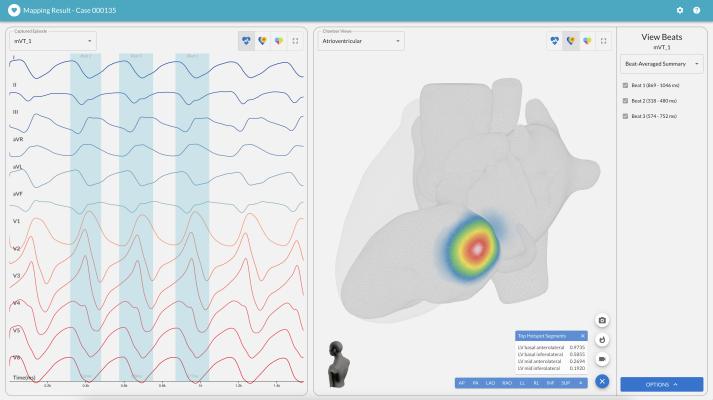
With advanced visualization and automation features, vMap empowers physicians to accurately identify the source location of cardiac arrhythmias. (Graphic: Business Wire)
September 6, 2023 — Vektor Medical, developer of the only technology to accurately map arrhythmias using just 12-lead ECG data, announced the release of a series of software enhancements to its AI-based non-invasive solution, vMap. Designed to improve ablation outcomes and procedural efficiencies, the newly updated vMap software integrates additional automation and advanced visualization features.
vMap is the only FDA-cleared, AI-based non-invasive solution that unlocks the data contained in a standard 12-lead ECG, enabling physicians to rapidly and accurately locate arrhythmia source locations. The software upgrades are designed to improve the accuracy, visualization, and efficiency of arrhythmia analysis and interpretation. The updates include:
Beat Assist: Software recommends and auto-selects arrhythmic beats of interest that may indicate the source location of an arrhythmia. This new functionality reduces uncertainty and increases confidence when identifying arrhythmic beats, speeding up procedure planning process.
Upgraded Heart Model: vMap’s new model provides a highly realistic and more detailed representation of the heart. It improves visualization and understanding of spatial distribution of the target lesion sites, increasing analysis accuracy and translation.
Automated ECG Baseline Correction: The revolutionary signal conditioning removes baseline noise from input ECG signals, improving accuracy and ultimately providing higher-quality ECG signals for analysis.
“The vMap software upgrades and enhanced visualization capabilities have made a significant impact on my ability to see the arrhythmia source location and plan the EP procedure,” stated Dr. Greg Feld, Director of Electrophysiology at UC San Diego Health. “The level of detail and clarity solidify vMap as a cutting-edge solution in arrhythmia care and will empower EPs to navigate the heart with more accuracy and confidence."
In addition, the upgrades include a series of workflow enhancements to simplify analysis, aid with pattern recognition and abnormality identification and reduce mapping time. These updates highlight Vektor Medical’s commitment to continuous innovation to transform arrhythmia care.
For more information: www.vektormedical.com


 January 15, 2026
January 15, 2026 









