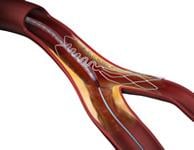
June 24, 2010 – Tryton Medical Inc. announced that the company’s Tryton Side Branch Stent System has been used in three cases in Israel for the first time.
Yaron Almagor, M.D., director of the interventional cardiology and cardiac catheterization laboratories at Shaare Zedek Medical Center in Jerusalem, Israel, performed the first implants.
“I am very pleased with the Tryton Stent System,” said Almagor. “Recently I had successful outcomes treating two patients with cases of complex true bifurcations – including one with left main disease, which can be very difficult to treat. I look forward to utilizing the Tryton stent to treat complex cases when it becomes commercially available for use in Israel.”
Left main disease, an accumulation of plaque that narrows the base of the coronary tree, until recently has been treated with bypass surgery. Recent studies have demonstrated that stents may provide physicians and patients with an important alternative. Widespread adoption of stenting for left main disease has been hampered by the lack of a robust strategy for bifurcations lesions, which represent more than 75 percent of left main lesions. A Tryton strategy for left main disease has the potential to provide an important tool as stenting becomes established as a standard treatment for left main disease.
The Tryton Stent System is currently in the process of obtaining regulatory approvals in Israel. The first three implants were performed under a special exemption approval by the Ministry of Health.


 November 24, 2025
November 24, 2025 









