Nov. 7, 2014 — Sorin Group, a global medical device company and a leader in the treatment of cardiovascular diseases, implanted the Solo Smart surgical replacement performed by David Heimansohn, M.D., at St. Vincent’s Heart Hospital in Indianapolis, Ind.
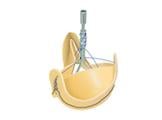
Solo Smart is the first and only valve with a removable stent to be approved in the U.S. market. This unique bioprosthesis, made from bovine pericardium, has no synthetic material added, allowing it to mimic the healthy native aortic valve. Solo Smart maximizes blood flow and delivers excellent hemodynamic performance, while providing the ease of implantability of a stented valve.
Solo Smart is the next generation of the Freedom Solo valve, which has been available in Europe since 2004.
For more information: www.sorin.com


 December 24, 2025
December 24, 2025 









