Append Medical, developer of a novel left atrial appendage (LAA) closure device to minimize stroke risk in atrial fibrillation (AF) patients, announced that it has been selected from among hundreds of startups as a finalist for the ICI Innovation Award Competition. It uses a transcatheter lasso system so no implant is left behind and eliminates the possibility of embolization.
December 18, 2019 — Append Medical, developer of a novel left atrial appendage (LAA) closure device to minimize stroke risk in atrial fibrillation (AF) patients, announced that it has been selected from among hundreds of startups as a finalist for the ICI Innovation Award Competition.
Append Medical will also present in the Digital Presentations: Complex Coronary and Valvular Interventions program during the meeting.
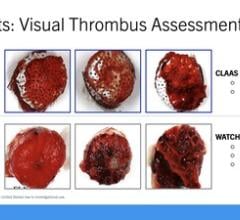
Current treatments for AF patients include anticoagulants and LAA closure devices, which are limited in effect due to high levels of non-compliance and bleeding risk (anticoagulants) and the risk of device leakage and device-related thromboembolism (closure devices).
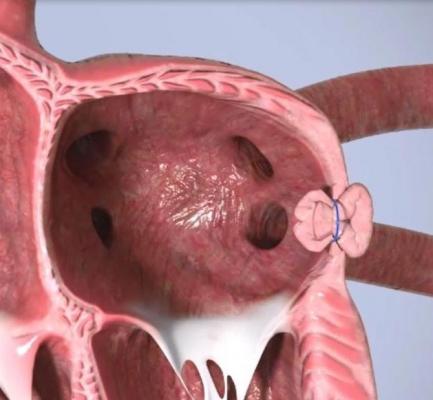
Append’s novel no-implant approach addresses these issues by closing the LAA with only a suture left at the closure site to prevent blood clot leakage and minimize device-related thromboembolism.
Originally conceived by Professor Leonid Sternik, MD, Director of the Department of Cardiac Surgery at Sheba Medical Center, the Append Medical “Appligator” device is designed to reduce stroke risk in AF patients by completely closing the LAA to prevent blood clot leakage. Its design is intended to minimize device-related thromboembolism risk by leaving only a suture – and no metal – at the closure site.
The LAA closure market is the second-fastest growing segment of the medical device market, estimated to reach $958 million by 2025, according to Energias Market Research.
The company operates as part of MEDX Xelerator in Israel. MEDX Xelerator’s partners include MEDX Ventures, Boston Scientific Inc., Intellectual Ventures and Sheba Medical Center.
For more information: wwwicimeeting.com.


 April 04, 2024
April 04, 2024