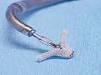
September 3, 2009 – Percutaneous cardiac valve maker Evalve Inc. announced treatment this week of the first group of patients with the MitraClip system at the Karolinska University Hospital in Solna, Stockholm, Sweden.
The MitraClip system is the only medical device commercially available in the European Union that provides a nonsurgical mitral valve repair option for patients suffering from the effects of functional and degenerative mitral regurgitation (MR).
MR is the most common type of heart valve insufficiency in Europe and the United States, affecting millions of people worldwide. Significant MR affects more than eight million people in the U.S. and Europe, the majority of which have FMR. There are more than 600,000 new diagnoses of significant MR each year in Europe and the U.S.; however, only 20 percent of these patients undergo surgery each year. Many higher risk surgical patients and non-surgical patients continue to be affected by the chronic volume overload caused by MR, which requires the heart to work harder, and may ultimately lead to heart failure.
The first patients in Sweden were successfully treated by a team led by cardio-thoracic surgeon Anders Jonsson, M.D., Ph.D., assisted by interventional cardiologist Magnus Settergren, M.D., Ph.D. Echocardiography was performed by cardiac anesthetist Jan Hultman, M.D., Ph.D. and clinical cardiologist Reidar Winter, M.D., Ph.D.
"The MitraClip technology has added a new dimension to the treatment of mitral regurgitation. In my initial experience with our first three patients, I was very pleased to see the good result achieved with a single clip in each of our patients. Any less invasive approach for heart valve procedures is worthwhile to consider, especially if the long term results are compatible with the techniques requiring an open thorax. I am looking forward to treat more patients to further evaluate this approach," said Dr. Hultman, director of the clinic in cardiothoracic surgery and anesthesia. "It is very satisfactory for us as a unit to be able to offer this technology to some of our patients. The MitraClip is a device that hopefully will enable us to design the optimal treatment for all different types of patients with MR including high risk and inoperable candidates," added Dr. Jonsson, who led the team.
Evalve initiated commercial sales of the MitraClip system in Europe under the CE mark in late 2008. The company is employing a direct sales strategy and is taking a disciplined and measured approach to the initial commercial roll out. Evalve has worked closely with hospitals to deliver high quality training programs in preparation for the first series of implants and offers ongoing support. The MitraClip device is currently being implanted in six Western European countries, including Germany, Italy, the United Kingdom, the Netherlands, Switzerland and now Sweden.
"We are very pleased with the successful initial use of the MitraClip device in Sweden at this outstanding cardiac center," said Ferolyn Powell, president and chief executive officer of Evalve. "It is noteworthy that the MitraClip therapy can be delivered to patients by both interventional cardiologists and cardiothoracic surgeons in close collaboration with their colleagues in echocardiology. The centers offering the MitraClip therapy in Europe are pioneering a new field and era in the medical care for patients who suffer from mitral regurgitation, especially for those who, until now, had very limited therapeutic options."
For more information: www.evalveinc.com, www.thefoundry.com


 December 24, 2025
December 24, 2025 









