January 13, 2010 – Transcatheter heart valve development company CardiAQ Valve Technologies (CVT) closed a $6.5 million funding deal to clinically validate its technology and carry the company through initial first-in-man studies.
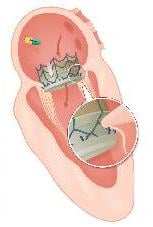
The company is developing a self-conforming, self-anchoring technology for its Transcatheter Mitral Valve Implantation (TMVI) system. The device is designed to treat functional mitral regurgitation (FMR) in patients who are often too sick to have surgery. The company hopes to show in trials it nonsurgical alternative could be more effective than surgical replacement.
Through the combination of a specialized anchoring mechanism and a new delivery catheter, CVT said its device will enable physicians to accurately and securely implant a new mitral valve within a beating heart. The CVT procedure is designed for use in a cardiac catheterization laboratory, resulting in less trauma to the patient and substantial cost-savings to the healthcare system.
The funding was led by Rob Michiels and the same group of investors who were the first to recognize the potential of percutaneous aortic valve replacement with their 2002 investment in CoreValve.
For more information: www.cardiaq.com


 December 24, 2025
December 24, 2025 









