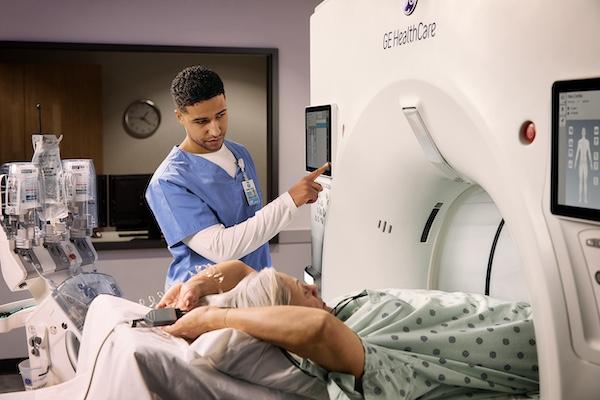
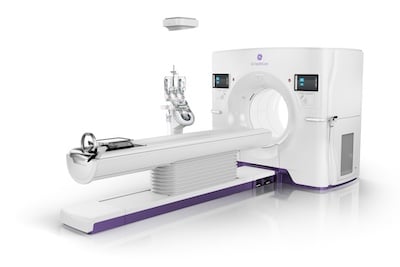
Sept. 2, 2025 — GE HealthCare recently received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Revolution Vibe CT system, an innovative imaging solution designed to elevate coronary CT angiography (CCTA). With Unlimited One-Beat Cardiac imaging and integrated AI-powered tools, Revolution Vibe offers clinicians a fast, powerful, and non-invasive way to identify potential heart blockages while enhancing patient comfort and streamlining workflow.
Cardiovascular disease (CVD) remains the leading cause of death globally, with projections estimating over 23 million deaths annually by 2030.[ii] As the demand for CCTA continues to rise, Revolution Vibe offers healthcare providers a powerful tool to meet this growing need. The system’s FDA clearance paves the way for broader adoption across U.S. facilities, enabling clinicians to deliver high-quality care even in the most challenging cases.
“Revolution Vibe’s FDA clearance comes at the perfect time, with guidelines being adopted clinically for cardiac CT and with positive reimbursement driving procedure growth, this is the time to usher in a new era of cardiac imaging,” shares Jean-Luc Procaccini, president and CEO, Molecular Imaging and Computed Tomography, GE HealthCare. “Designed to help clinicians detect coronary artery disease and help plan valvular heart interventions quickly and confidently – even in complex cases – Revolution Vibe offers a practical, high-performance solution to help improve diagnostic accuracy, reduce exam time, and support better patient outcomes.”
Revolution Vibe is engineered to address the most difficult cardiac exams, including patients with arrhythmias, heavily calcified coronaries and valves, stents, or bypasses. The system’s Unlimited One-Beat Cardiac imaging delivers full-heart clarity at low dose, even in cases without an ECG trace. Innovative features such as ECG-less Cardiac, TrueFidelity DL, SnapShot Freeze 2, and Effortless Cardiac Workflow optimize image quality and reduce exam time, helping make cardiac CT more accessible and efficient.
In clinical evaluations, Revolution Vibe with Effortless Workflow demonstrated a 50% reduction in exam time and up to five minutes saved in patient preparation per scan.i It also optimized CCTA exam scheduling, doubling CCTA capacity.i These efficiencies are driven by automated protocol selection, simplified positioning, and minimized reliance on beta blockers and ECG connections.
“The cardiac CT revolution is just beginning – and we are thrilled to be a part of it,” shares Sébastien Saint Germain, Director, Radiologie du Var – ELSAN, the first global healthcare institution to install GE HealthCare’s Revolution Vibe. “With Revolution Vibe in our fleet, we feel better prepared to meet growing demand for cardiac imaging and are proud to offer a fast, noninvasive diagnostic solution to our patients. In the first couple weeks of use, we’re already seeing how its AI-driven workflow and advanced imaging capabilities can help us deliver faster, more confident diagnoses.”
Radiologie du Var – ELSAN is a leading medical imaging provider in the Toulon region of southern France, operating across three sites: Polyclinique Les Fleurs, Clinique du Cap D’Or, and Clinique Saint-Michel. Known for its advanced imaging capabilities and commitment to innovation, the institution performs one of the highest volumes of cardiovascular imaging in the country.[iii]
With the addition of Revolution Vibe, Radiologie du Var – ELSAN now operates 10 imaging systems, including 1.5T and 3T MRIs, a spectral scanner, and several other impressive modalities. The organization’s mission is to ensure comprehensive and timely care for all pathologies by leveraging a networked approach—directing patients to the most appropriate equipment and expert teams for fast, accurate diagnoses.
Beyond cardiac imaging, Revolution Vibe supports general imaging needs and is designed to help facilities expand service lines while managing lifecycle costs. With energy-efficient design, Smart Subscription, fleet management, and extensive training, the system offers a future-ready solution for healthcare providers.
For more information on Revolution Vibe, visit gehealthcare.com.
[i] GE HealthCare case study. “Revolution Vibe: A new vision for heart at Radiomed.” Results may vary. The statements by GE HealthCare customers are based on their opinions and results achieved in their unique setting. Since there is no “typical” facility and many variables exist (i.e., size, case mix, etc.), there can be no guarantee that other customers will achieve the same results.
[ii] Cury, Ricardo C., Leslee J. Shaw, James K. Min, et al. “Coronary Artery Disease Detection by Multidetector CT Angiography: A Performance Meta-Analysis of 83 Studies.” Journal of the American College of Cardiology 60, no. 10 (September 2012): 913–21. https://doi.org/10.1016/j.jacc.2012.03.038.
[iii] ELSAN. “Radiologie du Var (83).” ELSAN, https://www.elsan.care/fr/radiologie-var. Accessed August 18, 2025.


 January 15, 2026
January 15, 2026 









