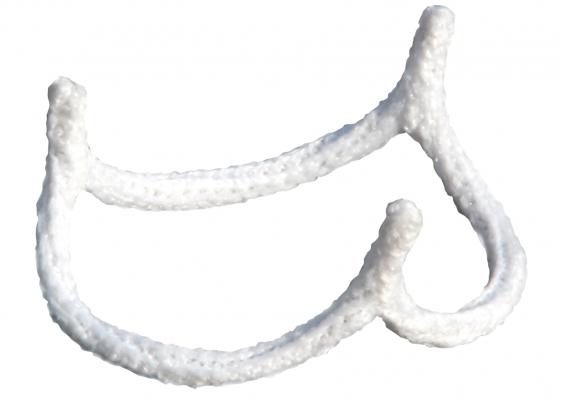
July 5, 2017 — BioStable Science & Engineering Inc. announced the first commercial uses of the HAART 300 Aortic Annuloplasty Device in the U.S. by doctors at the West Virginia University (WVU) Heart and Vascular Institute in Morgantown, W. Va. Lawrence Wei, M.D., Vinay Badhwar, M.D., and J. Scott Rankin, M.D., of the WVU Heart and Vascular Institute were the collaborating surgeons for the procedures. The WVU Heart and Vascular Institute is the first of a select group of leading U.S. centers expected to participate in the limited launch of the HAART 300 device this year.
Commenting on his experience, Wei, co-director of the WVU Center for Aortic Surgery said, ”The novel sizing method and simple, quick implantation technique for the HAART 300 Aortic Annuloplasty Device help to standardize the overall repair procedure. Internal annuloplasty has significant advantages over existing aortic valve repair techniques and makes aortic valve repair a more attractive treatment option for a broader group of patients.”
Badhwar, executive chair of the WVU Heart and Vascular Institute, added, “Our surgeons are committed to offering patients the latest medical technologies that have the potential to improve patient outcomes. We believe the HAART Aortic Annuloplasty Device fills a significant technological need that helps make aortic valve repair a simpler and more reproducible procedure to the benefit of patients.”
In March of this year, HAART 300 became the first aortic annuloplasty device to receive clearance from the U.S. Food and Drug Administration (FDA). BioStable’s second technology offering, the HAART 200 Aortic Annuloplasty Device for bicuspid aortic valve repair, is currently under regulatory review both in Europe and the U.S.
For more information: www.biostable-s-e.com


 December 24, 2025
December 24, 2025 









