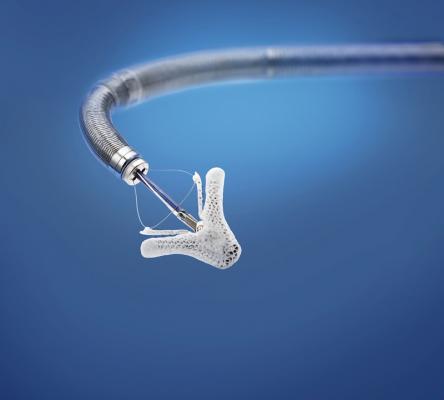
May 8, 2014 — Abbott announced its catheter-based MitraClip therapy has received Health Canada approval, providing physicians in Canada with the treatment option that can significantly improve symptoms, disease progression and quality of life for certain people with mitral regurgitation (MR).
The MitraClip device has been approved for people with degenerative MR who are too high risk for mitral valve surgery based on evaluation by heart experts. Degenerative MR is a type of MR caused by an anatomic defect of the mitral valve of the heart. Treatment with the MitraClip device can be effective in reducing the symptoms associated with severe MR, such as shortness of breath and fatigue, which may help people lead a more active lifestyle.
MR can raise the risk of irregular heartbeats, stroke and heart failure. Open-heart mitral valve surgery is the standard-of-care treatment, but many patients are too high risk for an invasive procedure. Medications for the condition are limited to reducing symptoms and do not have the ability to stop the progression of the disease.
"As cardiac surgeons, we often see patients with mitral regurgitation who are too frail or sick for mitral valve surgery and are severely limited in their daily activities due to their disease," said Gideon Cohen, M.D., Ph.D., FRCS, chief, division of cardiac surgery, Sunnybrook Health Sciences Centre, University of Toronto. "The MitraClip system provides a new catheter-based, minimally invasive treatment option for many of these patients, addressing an important unmet need and helping them regain their quality of life."
Multiple trials, published reports and registries of patients treated with the MitraClip device consistently demonstrate a positive safety profile, reduction in mitral regurgitation, improvement in symptoms and reduction in hospitalizations for heart failure.
"Patients treated with the MitraClip device can have dramatic improvements when their mitral regurgitation is reduced and their heart can pump blood more efficiently," said Anita Asgar, M.D., FRCPC, FACC, cardiologist, Montreal Heart Institute. "Many of these patients have suffered with mitral regurgitation without access to other treatment options, making the approval of the MitraClip device in Canada tremendous news as it offers a new alternative to these patients.”
Abbott's MitraClip repairs the mitral valve without the need for an invasive surgical procedure. The device is delivered to the heart through the femoral vein, and once implanted, allows the heart to pump blood more efficiently, thereby relieving symptoms and improving quality of life. Patients undergoing MitraClip treatment typically experience short recovery times and short hospital stays of two to three days.
"Health Canada approval of MitraClip marks an important milestone in bringing this first-of-its-kind therapy to many patients who have no other viable treatment options for their mitral regurgitation," said Charles A. Simonton, M.D., FACC, FSCAI, divisional vice president, medical affairs, and chief medical officer, Abbott Vascular. "We look forward to making this technology available to specialized centers in Canada with teams of heart doctors experienced in the management of patients with mitral valve disease, with the aim of providing people with the best possible treatment options."
For more information: www.abbott.ca


 December 24, 2025
December 24, 2025 









