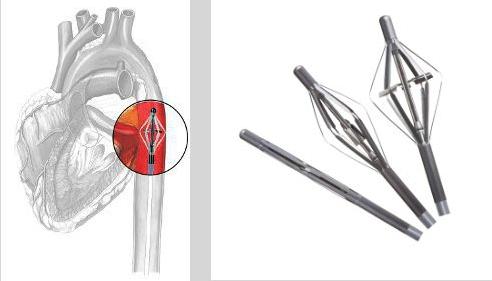
The Cardiobridge 10 French circulatory support catheter pump expands in the aorta to allows a propeller to increase blood flow in hemodynamically compromised patients.
March 27, 2013 — The Heart Failure 2013 LA meeting will include a showcase of several startup companies offering innovative new approaches to address heart and cardiovascular disease, most of them based in Southern California. The showcase starts at 5:30 p.m. at the event being held Saturday, April 6, at the Millennium Biltmore Hotel in Los Angeles.
Companies and technologies being highlighted include:
MyoStim Pacers — www.myostimpacers.com — World's first heart failure pacemaker with ability to recruit repairative stem cells to damaged and weakened heart tissue.
Bioheart Inc. — www.bioheartinc.com — Phase III leader in applying adult muscle stem cells to treat advanced heart failure. Only cell type known to grow new contractile muscle in the depths of scar tissue.
BioPace — World's first biological pacemaker made entirely of living cells.
CoroStim — World's first vibrational energy emitting pacemaker that prevents plaque formation in high-risk coronary arteries.
BioLeonhardt — www.bioleonhardt.com — Combination electrical stimulation and multi-stage cell and gene therapy method, composition and devices for treating advanced heart failure. Featuring combined utilization of MicroRNAs, nutrient time release SDF-1, hydrogel, cardiac stem cells, iPS cells and muscle progenitor stem cells. First method under testing with up to 36 repeat injection sessions of stem cells over time via a needle catheter.
Cardiobridge — www.cardiobridge.com — Highest flow rate 10 French circulatory support catheter pump in clinical testing for acute decompensating heart failure and high-risk PCI. Data on about 30 clinical patients will be presented.
AortaCell — Method and device to treat aortic aneurysms noninvasively with an abdominal belt that delivers focal wireless electrical energy to weakened aortic wall that causes repairative autologous stem cells to home to that chosen treatment location.
EndoCell — Method and device for growing new endothelium lining of a damaged artery with a percutaneous needle catheter and endothelial progenitor cells.
MyoValve — Cell seeding method and device for heart valve leaflets invivo or invitro. Designed to reduce risk of calcification and valve replacement.
Valvublator — Percutaneous catheter device for de-calcifying heart valves so a patient may keep their own valve instead of getting an artificial implant.
HeartScore — Composite scoring system of genetic, blood and scan test results (some point of care) that are loaded into the SecondBeat wearable wristwatch for a baseline score. The watch has an infrared sensor that measures real time endothelial health. The baseline score goes up or down if the patient eats well, exercises well and is in compliance with all doctor prescribed medicines and protocols. The composite HeartScore is clearly viewable to patients to help real time guidance of their heart and cardiovascular health.
For more information: www.leonhardtventures.com, www.calxcrowdfund.com, www.laheartfailure.com


 May 02, 2025
May 02, 2025 









