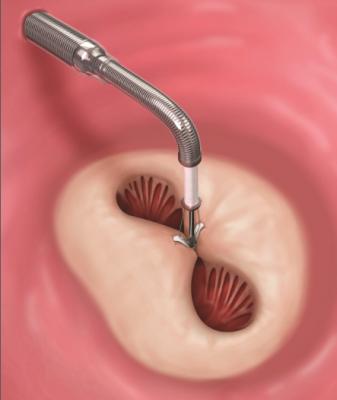
June 11, 2015 - Outcomes from the initial commercial experience of transcatheter mitral valve repair (TMVR) with Abbott's MitraClip were deemed favorable in a late-breaking clinical trial session at the American College of Cardiology's (ACC) 64 th annual scientific session and expo in March.
About three years ago, Betty Vaughn of Golden Valley, Minnesota, started to feel light-headed, fatigued and out of breath when she walked up and down the stairs. After visiting her doctor, the 89-year-old was diagnosed with degenerative mitral regurgitation (DMR), a heart condition in which the leaflets of the mitral valve do not close completely, causing blood to flow backward and leak into the left atrium of the heart. After her diagnosis, Paul Sorajja, M.D., cardiologist at Abbott Northwestern Hospital in Minneapolis, performed a TMVR procedure on Vaughn - who was not a good candidate for surgery - using Abbott's MitraClip in October 2014. Now, just months after the procedure, Vaughn has resumed many of the activities she loves, like working in the yard and playing cards with friends.
MitraClip treats people with DMR, which is a condition involving a dysfunction of the heart's mitral valve. It is a treatment option for people who are not good candidates for surgery, the current standard of care, because of their advanced age, frailty or other complicating factors. In the United States, mitral regurgitation (MR) is the most common valve disease, affecting nearly one in 10 people age 75 and older. People with MR often have difficulty with everyday activities, such as climbing stairs, and may require long periods of rest due to fatigue.
In the study, a transcatheter valve therapy (TVT) registry formed from a partnership with the Society of Thoracic Surgeons (STS) and ACC, researchers evaluated data from 564 people with TMVR who were treated with MitraClip following U.S. Food and Drug Administration (FDA) approval of the device in October 2013 through August 2014. The data continue to support the use of MitraClip, with successful treatment for 93 percent of people in the study. The treated group's median age was 83 years old, and they were not candidates for surgery because they were too frail or had other complicating factors. Sorajja, the lead investigator for this study, shared the data at the ACC session.
"The results of this MitraClip study are very favorable and consistent with the results we saw in studies prior to the therapy's U.S. approval," Sorajja said. "The results show clinically meaningful reduction in the severity of mitral regurgitation and improvement in the overall health of very sick people with prohibitive risk DMR who have no other meaningful options to improve their lives."
In the ACC presentation, Sorajja concluded that treatment with MitraClip for prohibitive risk patients with symptomatic MR is durable in a real-world commercial setting. Key findings from the 564 patients treated with the MitraClip device demonstrated the following:
- Prior to treatment, more than 90 percent of patients had a mitral regurgitation grade of 3 or 4, indicating significant leakage of the mitral valve. After treatment with MitraClip, 93 percent of patients achieved an MR grade of less than or equal to 2, with 63.6 percent at a grade of less than or equal to 1, demonstrating a significant decrease in leakage;
- Average length of stay in the hospital was 3 days, with 82 percent discharged home; and
- Adverse events and procedural complications were low, consistent with clinical trial experience.
For more information: www.mitraclip.com


 December 24, 2025
December 24, 2025 









