A comparison of intravascular ultrasound (IVUS) vs. angiography found a significant mismatch and showed the benefits of IVUS in venous interventions.
October 31, 2019 – In a large series of iliac vein stent cases, a blinded comparison found intravascular ultrasound (IVUS) superior to venography in determining the proper location of treatment zones. This is reported in the November 2019 edition of the Journal of Vascular Surgery: Venous and Lymphatic Disorders.[1]
Researchers from The RANE Center, St. Dominic’s Memorial Hospital, Jackson, Miss., performed a retrospective, single-center cohort study of 155 limbs treated for chronic iliac vein occlusion between 2013 and 2015.
“Adequate assessment of the location and degree of stenosis and delineation of venous anatomy for optimal landing zones are key elements in the success of interventions to treat chronic obstructions of the deep venous system,” noted lead author Myriam Montminy, M.D., MSc, FRCSC.
“While venography is more accessible and less expensive to perform than IVUS, an increasing number of studies demonstrate that IVUS is significantly more sensitive than venography in identifying stenotic lesions in the iliac-caval segments,” Montminy explained. “Our study aimed to take this one step further by comparing these modalities in identifying the key parameters required to guide stent placement.”
Key demographics of this series included:
• Age, years, mean (SD) - 59 (13)
• Male -30%
• Left leg - 61%
• Post-thrombotic - 72%
• Non-thrombotic - 28%
In the study, led by senior investigator Seshadri Raju, M.D., FACS, all of the cases utilized both venography and IVUS. Comparisons between the modalities were made in a blinded fashion.
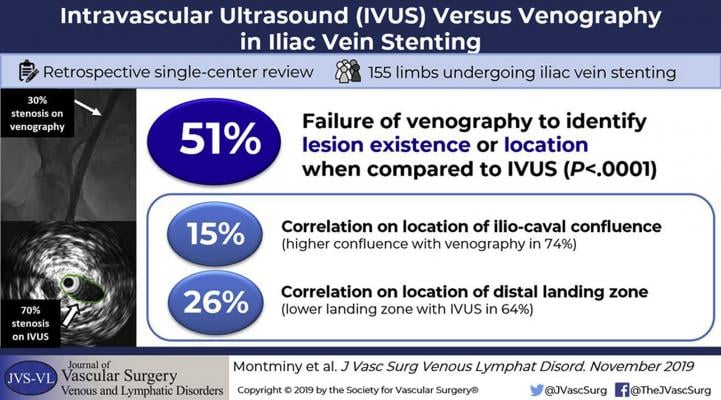
With regards to evaluation of the main venous stenosis, venography (compared with IVUS):
• Failed to identify the stenosis in 19% of cases
• Underestimated the degree of stenosis
• Failed to locate accurately the stenosis in 68% of cases
Further, in identifying the location of the iliac-caval confluence (the proximal landing zone), venography correlated with IVUS in 15% of cases, wherein IVUS revealed the confluence to be higher in 74% of cases (mean of one vertebral height higher).
Lastly, with regards to the distal landing zone, venography correlated with IVUS in 26% of cases, wherein IVUS located the optimal site lower in 64% of cases.
“This study highlights that venography compared to IVUS is likely to be deficient in all three areas of concern in venous stenting cases – location of the maximal stenosis as well as the optimal proximal and distal landing zones,” Montminy added.
Venography is still a desirable adjunct in iliac vein stenting as it provides a panoramic view of the pathologic process, including collaterals, she said. “Additionally, IVUS may miss or provide only a partial image of certain lesions situated at the hypogastric-iliac and iliac-caval confluences due to the absence of a centering mechanism.”
While it is currently unknown if the superiority of IVUS in identifying key parameters essential for iliac vein stenting translates into improved clinical outcomes, the results of this study further defines the complementary roles venography and IVUS play in this growing area of vascular intervention.
This research article is open source and free to the public until Dec. 31 at vsweb.org/JVSVL-IVUS.
Reference:


 January 15, 2026
January 15, 2026 









