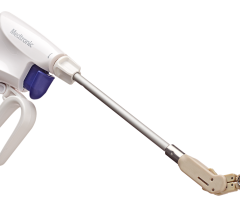
July 15, 2013 — Shore University Medical Center is one of only a handful of hospitals in the country offering the recent U.S. Food and Drug Administration (FDA)-approved Lariat Suture Delivery Device procedure. Electrophysiologists and interventional cardiologists perform this innovative procedure to help prevent stroke in patients who suffer from atrial fibrillation and are unable to take blood thinners.
“Atrial fibrillation is one of the most common heart rhythm problems seen in the world,” explains Dawn Calderon, D.O., FACC, chief of cardiology and director of the Adult Congenital Heart Center at Jersey Shore. “An estimated 2.7 million Americans suffer from atrial fibrillation, and these patients are five times more likely to suffer from stroke. For those who cannot tolerate traditional medications, the Lariat
Strokes typically occur when blood forms clots in the left atrial appendage (LAA), a small pouch which empties into the left atrium. Patients at high-risk for stroke are typically placed on oral anticoagulation therapy to prevent strokes; however some cannot tolerate these due to bleeding complications. In the past, these patients would require open-heart surgery. The Lariat procedure is a non-surgical option whereby the LAA is tied so clots cannot form, drastically reducing the risk of heart related stroke. Performed under general anesthesia, the procedure involves two catheters that are guided to the patient’s heart to seal the LAA with the device. Once in place, the LARIAT tightens a loop stitch around the base of the LAA, permanently sealing it off from the rest of the heart and closing it off to any blood flow.
According to Ashish Patel, M.D., electrophysiologist at Jersey Shore, “90 percent of strokes caused by atrial fibrillation originate from the LAA. The LAA provides no function, so when it is tied, the source of heart related stroke is reduced. Other benefits include less discomfort and a shorter recovery time than open heart surgery. The Lariat is truly a breakthrough for patients who can’t take blood thinners as it provides a new way to help prevent heart related stroke.”
Jersey Shore University Medical Center is one of the largest cardiovascular programs in New Jersey. Through Meridian CardioVascular Network, Jersey Shore offers the most complete, coordinated care from prevention and wellness programs to the diagnosis and treatment of heart disease to rehabilitation and recovery.
For more information: www.JerseyShoreUniversityMedicalCenter.com


 April 04, 2024
April 04, 2024