November 16, 2011 – Cambridge Consultants unveiled how it has collaborated with start-up company EBR Systems to develop the world’s first wireless pacing system. With cardiac stimulation leads considered the weak point in pacemaker systems, the Wireless Cardiac Stimulation system (WiCS) uses a leadless electrode to convert mechanical energy into electrical energy. The energy is wirelessly transmitted from an ultrasonic pulse generator and used to pace the heart as part of cardiac resynchronization therapy (CRT).
Eliminating the lead in the left side of the heart presents a major breakthrough in pacemaker design. It also brings new hope for more than 2.2 million advanced heart failure patients worldwide, who can benefit from CRT therapy. The new design provides a life-saving option for those patients who do not benefit from CRT therapy, either because of the complexity of the procedure or the limitations of the current technology.
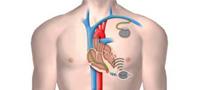
Current CRT pacemakers or defibrillators are implanted in patients with chronic heart failure requiring both the left and right ventricles to be paced. Such devices require the implantation of three leads. One of those involves painstakingly threading a lead through a difficult and complex route: running from the right atrium of the heart and into the coronary sinus—a vasculature structure on the outside surface of the heart—to the left ventricle.
A pacemaker/defibrillator device is connected to the leads, which are used to sense heart activity and deliver electrical stimulation through electrodes at the end of the leads. The electrical stimulation applied to the right and left ventricles synchronizes the heart’s contraction in a way that improves overall cardiac function in heart failure patients. However, added to the difficulty of the procedure itself is the chance of lead failure and infection.
WiCS overcomes these problems by leveraging advances in energy harvesting microelectronics. A very small leadless electrode is implanted in the desired location within the left side of the heart. In its first generation, WiCS works in conjunction with a conventional pacemaker/defibrillator, sensing the electrical pacing pulse of the pacemaker from the right ventricle. The pulse generator then transmits an ultrasonic pulse to the implanted receiver; the receiver converts the sonic energy into electrical energy to pace the left ventricle in synchronicity with the right.
This reduces the need for the difficult and complicated surgery associated with CRT pacemakers; by pacing inside the left ventricle, it also better mimics the natural activation and mechanical contraction pattern of the heart.
With hospital resources already stretched, the leadless system significantly reduces the time taken to carry out the complicated implantation procedure. The WiCS procedure is simple and predictable, resulting in streamlined scheduling of operating theaters and cost savings.
Using its knowledge of integrated circuit (IC) design, Cambridge Consultants helped EBR define the system architecture, which could be realized with modern silicon chip technology. Power consumption, size and safety are critical in pacemaker applications, and the team at Cambridge developed advanced low-power circuits to interface with the ultrasonic transducers and heart monitoring sensors.
With reliability critical, coupled with the need for sophisticated signal processing, the company created two state-of-the-art mixed-signal Application-Specific Integrated Circuits. Both devices are managed by an XAP4 processor. During the development, Cambridge created a full system hardware emulator of the final ASIC. This way, the other system elements and software could be designed and fully tested over a year before prototypes were available. This approach dramatically reduced the overall development time and enabled EBR to ensure the system would work as envisaged.
For more information: www.cambridgeconsultants.com, www.altran.com


 May 02, 2025
May 02, 2025 









