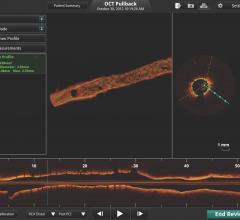
June 30, 2011 — The MemorialCare Heart and Vascular Institute (MHVI) at Long Beach Memorial has acquired the C7-XR OCT intravascular imaging system from St. Jude Medical. It is an imaging technology platform that aids physicians in the diagnosis and treatment of cardiovascular disease. Using optical coherence tomography (OCT), the system utilizes near-infrared light to create images that go beyond older coronary imaging technologies -- such as fluoroscopy and intravascular ultrasound (IVUS) -- offering cardiologists an assessment method with enhanced clarity for their patients with coronary artery disease.
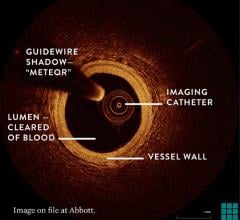
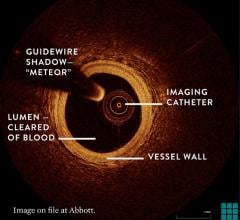
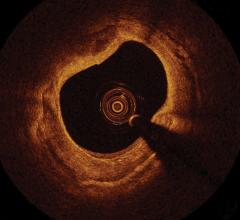
The system allows physicians at Long Beach Memorial to visualize and measure important vessel characteristics otherwise invisible or difficult to assess with previous intracoronary imaging technology. It can assist physicians in stent selection by providing them with precise measurements of lesion dimensions and vessel size and structure.
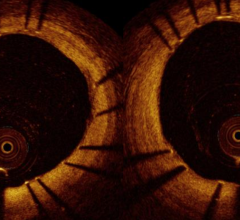
“The C7-XR System can be especially important for the assessment of stent placement because the high-resolution images show precisely how the stent is holding the artery open and whether it is positioned correctly against the artery wall, optimizing treatment and follow-up strategies,” said Rex Winters, M.D., medical director of invasive technology, MHVI.
In addition to guiding stent selection, the system provides physicians with post-stenting information, potentially minimizing the risk of repeat revascularization, where the patient would need to have another surgery to restore blood flow to an organ or tissue. At follow-up, the OCT technology provides detailed information regarding the inner lining of the vessel and whether there is a reoccurrence of the blood vessel narrowing.
The OCT technology platform creates images with 10 times the resolution of IVUS. The superior resolution and depth of focus found in OCT images aids in optimizing stent selection, evaluating stent apposition and the precise assessment of lesion morphology.
The C7-XR system with the C7 Dragonfly imaging catheter was launched in Europe in May 2009 and the M3 OCT imaging system had been the only commercially available intracoronary OCT imaging device in the world. The C7-XR is commercially available in the United States.
For more information: www.sjm.com/

 August 29, 2023
August 29, 2023