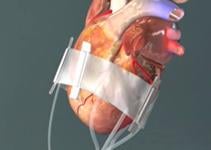
September 29, 2009 – Mardil Inc. last week at TCT 2009 announced positive interim data from a pilot study investigating the safety and efficacy of its novel cardiac device – BACE (Basal Annuloplasty of the Cardia Externally) – in treating mitral valve regurgitation.
The first 11 patients implanted with BACE demonstrated a significant reduction in the severity grade of their mitral regurgitation, from a baseline mean grade of 3.32 to a mean grade of 0.61 post implantation, the data demonstrate. Mitral regurgitation severity is graded on a scale from zero to four, with four representing the most severe condition. The improvements in mitral valve function were sustained at six months, as demonstrated by follow-up echocardiograms conducted in the three of the 11 patients. No device related adverse events were reported.
"While the data are preliminary, they represent a level of improvement that is extremely encouraging," said Jai Raman, M.D., Ph.D., professor of surgery and director of adult cardiac surgery and cardiothoracic surgical research at the University of Chicago. "BACE represents a novel modality for treating functional mitral valve regurgitation because it addresses the root cause of the condition - a heart muscle that is enlarged and weakened – whereas current devices on the market attempt to replace or repair valves that are structurally normal."
The pilot study of 20 patients was designed to assess the safety and efficacy of the BACE Device, a less invasive cardiac device that sits outside the heart and supports the weakened ventricular muscle while treating valvular dysfunction. Eleven patients with moderate to severe ischemic mitral regurgitation underwent implantation with BACE, along with coronary artery bypass grafting (CABG) on a beating heart; seven of them underwent surgery without a heart-lung bypass machine. Three patients had left ventricular reconstructive procedures. One patient died of complications related to insertion of a mechanical support device that was placed pre-operatively. No device-related adverse reactions were reported in the trial.
The pilot study is being conducted in India. Mardil is seeking an investigational device exemption (IDE) through the U.S. Food and Drug Administration to begin clinical trials in the U.S. next year.
For more information: www.mardil.com


 February 06, 2026
February 06, 2026 









