Mayo Clinic is using artificial intelligence EchoGo Core software from Ultromics to automatically analyize echocardiography images to assess the cardiac impact of COVID-19.
May 19, 2020 — A joint U.K.-U.S. research team is applying a pioneering artificial intelligence (AI) system to automatically analyze cardiac ultrasound imaging to map for the first time how the COVID-19 (SARS-CoV-2) virus attacks the heart with such deadly impact.
The coronavirus has been found to kill more than 1 in 10 victims with heart disease and also to significantly weaken the hearts of other sufferers.
This week, British health-tech company Ultromics and Mayo Clinic in the U.S. will use AI software, EchoGo Core, to analyze echocardiograms of COVID-19 victims, for clues about how the virus affects the human cardiovascular system.
Their findings will produce, for the first time, a map of the novel cardiac features of COVID-19 and help physicians rapidly triage and treat high-risk patients, potentially saving countless lives.
"To date, there is no way of linking the impact of the virus to predicted patient outcomes," Said Ultromics CEO Ross Upton. "By applying our technology to the evaluation of COVID associated echocardiograms, we can help understand the characteristics of cardiac involvement. We hope that by discovering a way to do this, patient management can be optimized – this is incredibly important where resources are scarce. Most importantly, we can give physicians the gift of time to treat those most in danger."
Mayo Clinic is one of the world's leading centers of cardiology and its extensive cardiac knowledge will assist Ultromics in the development of an image analysis application to help clinicians in the fight against COVID-19. The collaboration will be led by Gary Woodward, CTO of Ultromics and Patricia A. Pellikka, M.D., cardiologist, and clinical researcher at Mayo Clinic.
The COVID-19 coronavirus has considerable potential for cardiovascular impact including COVID induced microvascular disease and myocarditis, and side-effects from some treatments, known as therapy-associated cardiotoxicity.
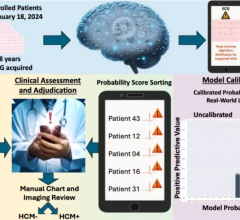
The multi-site study will look at 500 COVID-19 positive men and women, aged between 18 and 89. These participants will have undergone a clinically indicated echocardiography exam during a three-month period. The primary objective is the assessment of automated cardiac measurements, ejection fraction and global longitudinal strain, for the classification of COVID-19 patient outcomes.
EchoGo Core can provide physicians with an alternative streamlined solution for monitoring and identifying heart disease, enabling healthcare providers, no matter what experience level, to perform analysis with ease. This could be hugely important in giving physicians freed time to provide high quality, patient-centric care.
Echocardiograms have a proven role in the identification and assessment of virtually all cardiac disease entities. The non-invasive method is cost-effective and widely available, ideal for bedside assessment of patients with suspected cardiac complications of COVID-19.
What Does the EchoGo Core AI Software Do?
EchoGo Core applies artificial intelligence to automate the analysis on echocardiograms. The system has been validated with the National Health Service (NHS) in the U.K., in one of the largest echo studies of its kind, with over 8,000 patients in over 30 sites. It is a fully automated, zero-click system which has zero variability, so it allows physicians, regardless of skill level, to make an efficient and accurate diagnosis.
Ultromics applies artificial intelligence to develop echo-based tools in cardiovascular imaging, by combining deep clinical insight with machine learning and some of the largest echo datasets in the world. The revolutionary platform assists clinicians in their decision-making, without disrupting clinical workflow, to drive diagnostic quality and patient care.
EchoGo Core is FDA-cleared for use in the United States, not CE marked for use in the European Union.
For more information: www.ultromics.com


 September 24, 2025
September 24, 2025