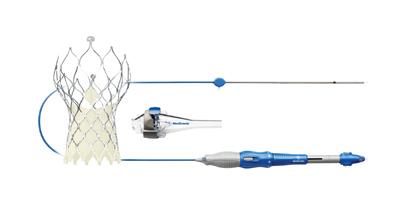
June 9, 2015 - Medtronic plc announced initial clinical outcomes for its next-generation CoreValve Evolut R System at the 64th annual scientific session of the American College of Cardiology, March 14-16 in San Diego. At 30-days, the recapturable, self-expanding valve showed no incidents of all-cause mortality or stroke in a high and extreme risk patient population. The Evolut R Study enrolled 60 patients from six centers in the United Kingdom, Australia and New Zealand.
The system is not approved for commercial use in the United States, where it is currently undergoing clinical trials.
"Initial clinical experience with the Evolut R system is remarkable and ushers in a new era of TAVR [transcatheter aortic valve replacement] technology that provides increased confidence with recapturability, excellent procedural results and impressive clinical outcomes," said Ian Meredith, M.D., of Monash Heart - Monash Health, Melbourne, Australia, who is a co-principal investigator of the study. "The 14 French equivalent delivery system allowed transfemoral access for most patients and the recapturable technology enabled implanters to optimize valve placement for improved annular sealing and reduced conduction disturbances without compromise on mortality or stroke."
The EnVeo R Delivery Catheter System provides a new InLine Sheath that significantly reduces the profile to a 14 Fr equivalent, less than 1/5 inch. Transfemoral access was possible in all but one patient (98.3 percent) in a population that included patients with vasculature as small as 5mm diameter. All recapture attempts were performed safely with zero strokes (0.0 percent). Additionally, correct valve position with one device was achieved in 98.3 percent of patients, and there were no cases of valve dysfunction, procedural death, annular rupture, coronary occlusion, valve embolization or conversion to surgery in the study. The pacemaker rate was low at 11.7 percent.
The supra-annular valve design of the CoreValve Evolut R System resulted in low, single-digit mean aortic gradients (a commonly used threshold of exceptional blood flow) at each follow-up visit (9.2mm Hg in early follow-up at 24hrs to 7 days post-procedure, and 8.1mmHg at 30 days). The valve features a new extended sealing skirt on the 26 and 29 mm sizes that further promotes valve sealing at the annulus, resulting in low rates of paravalvular leak (PVL) with 96.6 percent of patients having ≤ mild PVL at 30-days.
The 23 mm, 26 mm and 29 mm sizes of the CoreValve Evolut R transcatheter valve and the CoreValve EnVeo R Delivery Catheter System are available in Europe and other countries that recognize the CE mark.
For more information: www.medtronic.com


 December 24, 2025
December 24, 2025 









