May 1, 2023 — Medtronic plc, a global leader in healthcare technology, announced it has received U.S. Food and Drug Administration (FDA) approval of its Micra AV2 and Micra VR2, the next generation of its industry-leading miniaturized, leadless pacemakers.
Micra AV2 and Micra VR2, the world’s smallest pacemakers, provide longer battery life and easier programming than prior Micra pacemakers, while still delivering the many benefits of leadless pacing such as reduced complications compared to traditional pacemakers.2
With approximately 40% more battery life compared to previous generations,1 the median projected battery life of Micra AV2 and Micra VR2 is nearly 16 and 17 years, respectively.3 This means more than 80% of patients who receive a Micra are projected to only need one device for life.3
The new Micra AV2 also includes advanced algorithms that automatically program AV synchrony, thereby coordinating the heart’s upper and lower chambers. Also, for patients who are active, Micra AV2 has a higher available tracking capability for faster heart rates (increased from 115 to 135 beats per minute for upper limits).4
“Improved AV synchrony – requiring less in-office reprogramming thanks to algorithm optimization – and longer battery life are major wins for patients,” said Camille Frazier-Mills, M.D., MHS, an electrophysiologist at Duke University Health System. “I’m excited to offer my patients the new Micra devices. This best-in-class technology transforms the patient experience by eliminating pocket-related complications, and now reduces the chance that patients will need their device changed in the future.”
“Our goal is to improve the patient experience by continuously reinventing our ground-breaking leadless pacemaker,” said Robert C. Kowal, M.D., Ph.D., general manager, Cardiac Pacing Therapies within the Cardiac Rhythm Management business, which is part of the Cardiovascular Portfolio at Medtronic. “Since inventing the first battery-operated cardiac pacemakers 65 years ago, Medtronic has transformed pacing technologies to benefit patients, including the nearly 200,000 patients globally who have received a Micra device so far.”
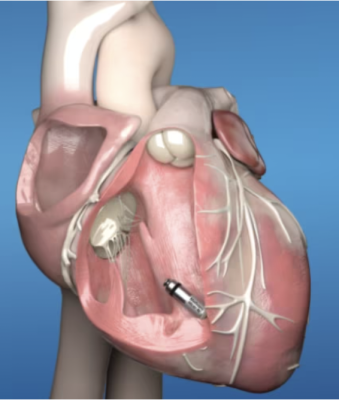
Comparable in size to a multi-vitamin, Micra pacemakers are less than one-tenth the size of traditional pacemakers. Unlike traditional pacemakers, Micra pacemakers do not require leads or a surgical "pocket" under the skin, so potential sources of complications related to leads and pockets are eliminated, and there is no visible sign of the device.
Leaders in Cardiac Pacing
This FDA approval builds on the Medtronic legacy in cardiac pacing, which includes:
- Inventing the first wearable pacemaker in 1957.
- Launching the first pacemaker for use in MRIs in 2011.
- Creating and launching the first leadless pacemaker in 2015.
- Launching the first leadless pacemaker to coordinate the heart’s electrical signals by sensing atrial activity, without a lead or device in the atria, in 2020.
- Gaining regulatory approval for the first pacing system to activate the heart’s natural electrical system, conduction system pacing (approval via the His-bundle in 2018 and via the left bundle branch area in 2022).
For more information: www.medtronic.com


 February 03, 2026
February 03, 2026 









