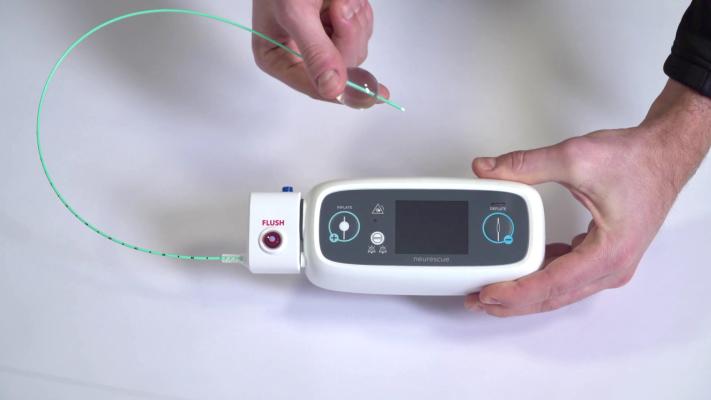
Image courtesy of Neurescue
May 13, 2022 — The MemorialCare Heart & Vascular Institute at Long Beach Medical Center has been designated the only national site to offer and enroll patients in the ARISE-Study to help increase survival rates for those who experience in-hospital cardiac arrests. The study uses the Neurescue device – a catheter-based balloon that is inserted through the groin into the aorta – to help improve circulation and blood pressure, while standard treatment happens in parallel. When the balloon inflates, it helps keep blood in the upper part of the body to circulate to the heart and the brain. This improved circulation and blood pressure can improve efforts to restart the heart.
"Seventy-five percent of patients with cardiac arrest die because they don't respond to current standard treatments of cardiopulmonary resuscitation (CPR), defibrillation and medicine," says David Shavelle, M.D., medical director, adult cardiology and interventional lab, MemorialCare Heart & Vascular Institute, Long Beach Medical Center. "This device gives us an extended chance at saving a life."
The ARISE-study is designed to see if this device can help a patient in cardiac arrest who has already received eight minutes of standard treatment but has not responded to it. Only physicians who've been trained in implanting this device will be allowed to use it during a cardiac arrest code. The patient may be enrolled in the study at that time. Since the risk of death after eight minutes of unsuccessful cardiac arrest treatment is very high, the investigators of the study believe the benefit of the device will outweigh any risk of device placement.
"The potential results of this study and device could fundamentally change how we treat and manage cardiac arrests in the hospital setting," said Shavelle. "If we are able to augment our standard treatment protocols to give cardiac arrest patients a greater chance of restarting their heart, this could really be a game changer and an advance desperately needed in the medical community."
If the medical team is able to restart the patient's heart with the device, the device would be left in place and the balloon deflated. The tube and balloon will be removed within an hour and the patient will be closely monitored in the cardiac intensive care unit.
The device is currently approved by the Food and Drug Administration (FDA) for use in patients with severe bleeding and is being used. throughout the U.S. for this indication.
For more information: memorialcare.org/LongBeach


 February 03, 2026
February 03, 2026 









