March 7, 2013 — Mercator MedSystems Inc. announced it has received CE mark approval to market its Cricket and Bullfrog Micro-Infusion Catheters in Europe, significantly expanding the market for these products, which have previously received 510(k) clearance from the U.S. Food and Drug Administration (FDA). The Mercator micro-infusion catheters are capable of non-systemic delivery of therapeutic agents directly across any peripheral or coronary blood vessel. Initially, these products will be commercialized for the treatment of peripheral artery disease (PAD), a critical health issue affecting 12-14 percent of the general population and nearly 20 percent of those over the age of 70. Approximately 23 million Western Europeans and 17 million Americans have PAD.
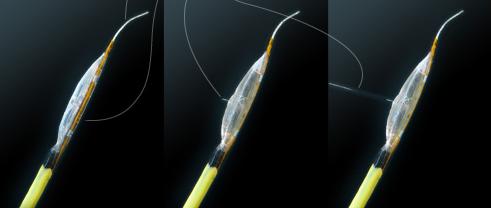
The Cricket and Bullfrog micro-infusion catheters establish a revolutionary platform that, for the first time, allows clinicians to accurately pinpoint the delivery of drugs and biologics deep into the body to treat the root cause of disease. Mercator’s patented, proprietary catheters can be guided to therapeutic targets, where a balloon, inflated with low pressure to prevent damage to the vessel wall, self-adjusts to the diameter of the vessel. It then deploys a micro-needle into the outer active tissue layer of the vessel wall, or adventitia — where the drugs are diffused to bathe the entire vessel cylindrically, from the outside to the inside, creating a unique, tissue-based, drug-eluting reservoir.
The adventitia as a novel therapeutic target has been well described in the scientific literature. In 2007, Kathryn Maiellaro-Rafferty, Ph.D., and W. Robert Taylor, M.D., Ph.D. from Emory University focused on the “outside-in” mechanism in the journal, Cardiovascular Research. Referencing cardiovascular disease pathologies, they noted, “Considering the outside-in hypothesis, we see that…the inflammatory events appear to initiate in the adventitia.” In reference to his ongoing PAD clinical research, Christopher Owens, M.D., MSc, associate professor of vascular and endovascular surgery at the University of California, San Francisco said, "The Bullfrog catheter has demonstrated its ability to efficiently deliver potent therapeutic agents outside the blood vessel, specifically to the adventitia. I believe we are now seeing the promise of adventitial delivery: a biologic effect of the drug interrupting the inflammatory cascade that normally leads to restenosis after a vascular intervention."
“By leveraging the adventitia’s biological properties, our game-changing technology minimizes drug dilution in the bloodstream as well as complications associated with systemic administration,” said Kirk P. Seward, Ph.D., Mercator president and chief technology officer. “This significantly reduces the inflammatory cascade, drug side effects and health care costs, while vastly improving therapeutic effectiveness and patient outcomes,” he added.
“The CE mark allows Mercator to begin changing the very nature of PAD therapy in Europe,” said Executive Chairman Thomas M. Loarie. “We are particularly excited about the great potential of our new paradigm of an ‘outside-in’ drug delivery system which can be rolled out in a variety of other applications — including hypertension, autologous stem cell delivery, bronchial therapy and lung cancer.”


 October 28, 2025
October 28, 2025 









