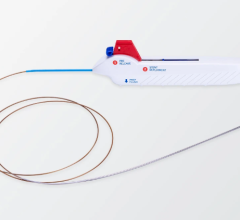
LARIAT Suture Delivery Device
August 24, 2009 – Physicians at The Mount Sinai Medical Center were the first in the country to perform a nonsurgical procedure using sutures to tie off a left atrial appendage (LAA), which is the source of blood clots leading to stroke in patients with atrial fibrillation (AFib). AFib is the most common sustained heart-rhythm disorder in the United States.
The procedure was performed Wednesday by Vivek Y. Reddy, M.D., professor of medicine and director of the cardiac arrhythmia service at Mount Sinai Heart, and his colleague, Srinivas R. Dukkipati, M.D., director of Mount Sinai’s experimental electrophysiology laboratory. With the patient under general anesthesia, the physicians guided two catheters into the patient’s heart to seal the LAA with a pretied suture loop. The technique is a safe alternative to drug therapies such as the blood thinner warfarin (Coumadin) that can have serious side effects, as well as open-heart surgery, and more invasive implant surgery.
“People who take Coumadin because of atrial fibrillation include active and otherwise healthy people, as well as elderly people for whom the drug may be contraindicated,” said Valentin Fuster, M.D., Ph.D., director of Mount Sinai Heart and chair of the American/European Guidelines of Atrial Fibrillation.
Drs. Reddy and Dukkipati joined Mount Sinai this month to focus on building the institution’s services for heart-rhythm disorders. They had been performing preclinical testing of the nonsurgical LAA device, and this procedure represents its first application in people in the United States.
“Compared to a lifetime of medication therapy, or other surgical modalities, a one-time, non-surgical procedure to relieve the complications of AFib offers a whole new paradigm,” said Dennis S. Charney, M.D., Anne and Joel Ehrenkranz Dean Mount Sinai School of Medicine, and executive vice president for academic affairs, The Mount Sinai Medical Center. “Drs. Reddy and Dukkipati have ushered in a new standard of care for people with this serious cardiac condition.”
Approximately 6 million U.S. adults have been diagnosed with AFib, a condition characterized by a rapid and irregular heartbeat that can cause serious complications, including stroke and early death. The majority of these patients take warfarin because left untreated, AFib can cause life-threatening blood clots. Approximately 25-30 percent of patients with AFib have contraindications to the drug, such as concerns of falling or imbalance in elderly patients and, even for the remainder, only about 55-60 percent receive warfarin.
AFib-related deaths have increased over the past two decades and now account for one-quarter of all strokes in the elderly. Those who do take warfarin must rigorously manage the drug’s level in their blood. High levels can cause excessive or internal bleeding, even after minor falls, bruises, or cuts. For some, this management regime can mean monthly tests over the course of many years. In eliminating the need to take warfarin, the LAA procedure can reduce the need for frequent medical visits.
“This nonsurgical procedure could lead to a permanent means of protecting against stroke in patients with AFib who are ineligible for long-term warfarin anticoagulation therapy,” said Dr. Reddy. “The suture delivery system allows us to place a pretied suture loop on the outside of the LAA using a pericardial approach, similar to what is used in surgery. But instead of surgery, which can involve spreading the ribcage or cutting through bone to access the LAA, this procedure does not require surgical incisions and is instead performed percutaneously; that is, using needle punctures to introduce catheters to the heart.”
In addition to open-heart surgery, which is rarely performed as a stand-alone surgical procedure because of the associated morbidity, nonpharmaceutical treatment options involve occlusion of the LAA via an implant, a technique that is not yet approved by the FDA, said Samin K. Sharma, M.D., director of Mount Sinai’s Cardiac catheterization laboratory.
The patient, a 78-year-old woman from Miami, presented with a history of stroke and a fall. Her physicians prescribed Coumadin but had difficulty titrating the medication for her. She initially sought an implant, but traveled to Mount Sinai for the nonsurgical, catheter-based suture delivery system. Following the procedure, the patient is recovering safely and no longer needs to take the drug for stroke prevention. Patients receiving noninvasive procedures usually return to normal activities in about a week.
The procedure, aided by the LARIAT Suture Delivery Device developed by SentreHEART, Inc. and approved by the FDA in May, was performed in a cardiac catheterization laboratory and did not require cardiopulmonary bypass.
For more information: www.mountsinai.org


 February 02, 2026
February 02, 2026