
August 12, 2020 — A national survey conducted by the patient advocacy group Heart-Valve-Surgery.com with support by Medtronic, found that nearly half (49 percent) of heart valve patients said returning to active living is a key factor in their treatment decision. The survey of more than 3,400 heart valve patients and caregivers uncovered real-world insights around managing heart valve disease, from diagnosis through treatment and beyond. The survey findings help kick off a new Active Living Awareness Initiative.
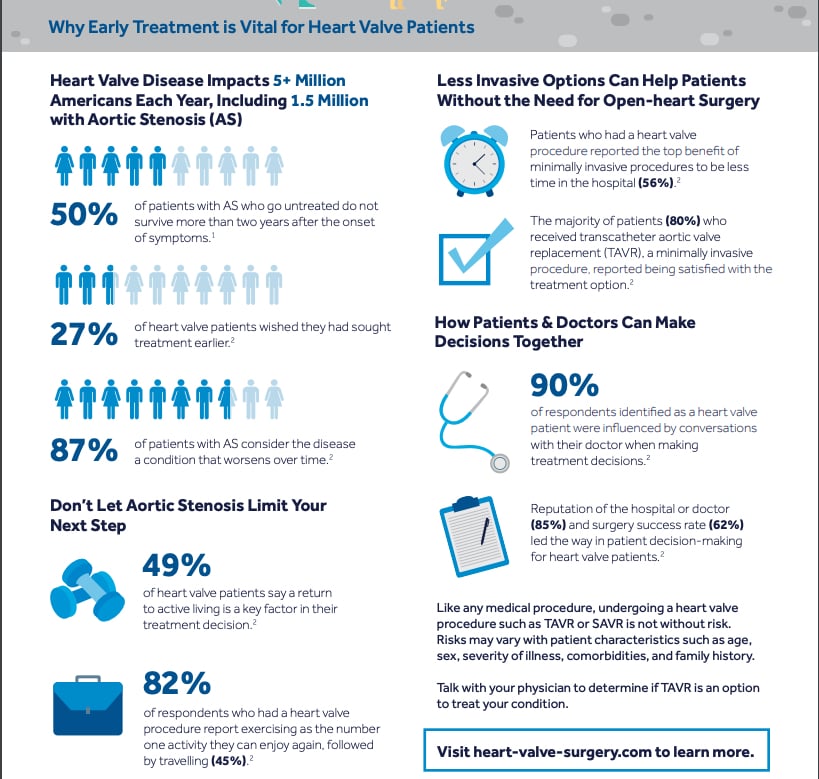
The initiative comes at a time when heart valve disease impacts more than five million Americans, including 1.5 million with aortic stenosis (AS). Considered to be one of the most common and severe forms of heart valve disease, AS occurs when the aortic valve narrows, preventing the leaflets from properly opening and closing, and diminishing blood flow between the heart and the rest of the body. In fact, 50 percent of patients with AS who go untreated do not survive more than two years after the onset of symptoms.
Despite the prevalence and severity of AS, the American Heart Association (AHA) warns that the COVID-19 pandemic has put health care on hold for many individuals leading to heart health emergencies.
For patients who are candidates, minimally invasive procedures, such as transcatheter aortic valve replacement (TAVR), generally require less time in the hospital with faster procedure times, and may result in a quicker recovery.
"From my personal experience as a heart valve patient and in helping millions of patients fight heart valve disease, I know how critical it is to be an informed patient and seek treatment before the disease becomes fatal," said Adam Pick, patient advocate and founder of Heart-Valve-Surgery.com. "Now more than ever, patients should feel empowered to take a meaningful and decisive role in their treatment decision-making process. We learned through this survey that many patients want to be active again – whether that means going back to work, spending more time with family and friends, or simply feeling well enough to walk up the stairs or to the mailbox."
The survey found that most respondents (90 percent) who identified as heart valve patients are more influenced by conversations with their doctor when making treatment decisions, while other top factors included conducting their own online research (55 percent) and having conversations with family or friends (28 percent). As the option of TAVR expands to new patient populations, a shared decision-making approach may be the best option for all patients considering aortic valve replacement, according to a March 2020 paper published in the Journal of the American College of Cardiology (JACC).[1]
"With the majority of heart valve patients reporting conversations with their doctors as the most important factor in their treatment choice, the importance in seeking treatment early and having an informed dialogue with their physicians, cannot be overstated," said Kendra Grubb, M.D., surgical director with the Structural Heart and Valve Center at Emory Healthcare in Atlanta. "These conditions need to be taken seriously early on, and we want patients to know that meeting with their doctor and considering all the factors is a vital first step in getting healthy again."
Additional key findings from the survey include:
• More than one-fourth of heart valve patients (27 percent) wished they sought treatment earlier.
• Reputation of the hospital or doctor (85 percent) and surgery success rate (62 percent) led the way in patient decision making for heart valve patients.
• The majority (87 percent) of patients with aortic stenosis consider the disease a condition that worsens over time
• The majority of respondents who had a heart valve procedure reported exercising as the number one activity they are able to enjoy again (82 percent), followed by working full- or part-time (46 percent) and travelling (45 percent).
• Patients who had a heart valve procedure reported the top benefits to minimally invasive procedures as less time in the hospital (56 percent) and a faster return to an active lifestyle (55 percent). The majority of patients who received TAVR (98 percent) reported being satisfied with TAVR as a treatment option.
The Active Living Awareness Initiative aims to educate on the importance of early treatment and diagnosis for heart valve disease, including AS. Key findings from the Active Living survey are available via an interactive microsite at Heart-Valve-Surgery.com. The microsite provides visitors video content about AS and its treatment, educational references including a Physician Finder, and free downloads including a checklist of "Aortic Stenosis: 5 Questions to Consider Before Treatment."
Explore the Active Living microsite.
About the Heart Valve Surgery Survey
These results are based on an online survey issued to the Heart-Valve-Surgery.com community, the world's largest educational resource and community of heart valve patients. More than 3,400 responses were received from heart valve patients and caregivers. The respondents were a 50/50 male female split with 72 percent over the age of 60 and 91 percent over the age of 50. Of the respondents, 94 percent identified as heart valve patients, 53 percent identified as aortic stenosis patients.
About Adam Pick and Heart-Valve-Surgery.com
Adam Pick is a heart valve patient and author of The Patient's Guide To Heart Valve Surgery. In 2006, Adam founded Heart-Valve-Surgery.com to educate and to empower patients from diagnosis through recovery. This award-winning website, which is sponsored by over 40 cardiac centers, has helped more than 10 million people fight heart valve disease. Adam has been featured by the American Heart Association and is currently followed by over 300,000 people across social media.
Reference:


 December 24, 2025
December 24, 2025 









