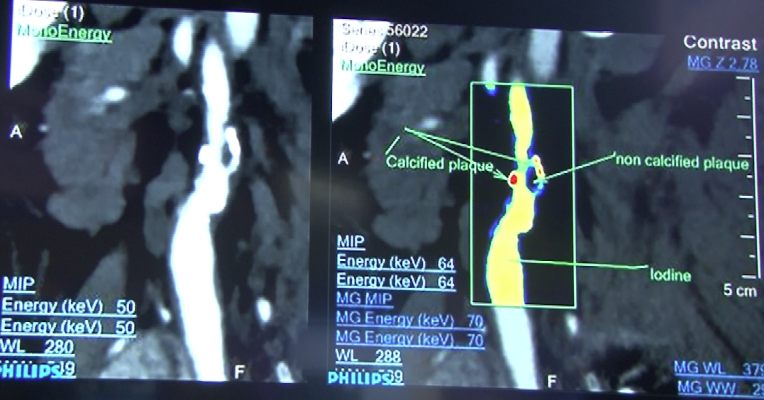
July 30, 2015 — Navidea Biopharmaceuticals Inc. announced plans to move forward with a joint study of the ability of Tc99m-tilmanocept, a Manocept platform product, to localize in high-risk atherosclerotic plaques. The study — funded through a $321,000 Phase 1 Small Business Innovation Research (SBIR) grant from the National Heart, Lung and Blood Institute (NHLBI), National Institute of Health (NIH) — will be conducted with Massachusetts General Hospital (MGH) and Harvard Medical School.
The specific plaques being studied are rich in CD206-expressing macrophages and are at high-risk for near-term rupture, resulting in myocardial infarctions, sudden cardiac death and strokes, accumulatively the leading cause of death in the United States.
The consequences of atherosclerosis and subsequent cardiovascular disease (CVD), while severe in all populations, are particularly concentrated in HIV+ patients. “This Phase 1 pilot study may provide evidence that Tc99m-tilmanocept localizes in atherosclerotic plaques which would enable potential development of a diagnostic imaging agent that could benefit not only HIV+ patients but millions of people,” said Michael Tomblyn, M.D., Navidea’s chief medical officer.
“HIV infected patients suffer disproportionally from atherosclerosis and CVD,” commented Steven Grinspoon, M.D., professor of medicine at Harvard Medical School, director MGH Program in Nutritional Metabolism, co-director of the Nutrition Obesity Research Center at Harvard and the study’s lead investigator. “There exists a profound and highly significant unmet need for means to better diagnose and treat atherosclerosis in all patients but particularly so in HIV patients. Manocept’s proven specificity for CD206 presents a clinically exploitable target with potential to achieve significant diagnostic and therapeutic results where few other approaches have progressed.”
Recently, it has been observed that CD206-expressing macrophages densely populate vulnerable plaques or thin cap fibroatheromas (TCFA) but not other kinds (i.e., stable) of atherosclerotic plaques. A primary goal for this grant involves an approved clinical investigation of up to 18 individuals with and without aortic and high risk coronary atherosclerotic plaques, and with and without HIV infection to determine the feasibility of 99mTc-tilmanocept to image high-risk plaque by single-photon emission computed tomography (SPECT)/CT. Results have the potential to provide evidence of the potential of 99mTc-tilmanocept to accumulate in high-risk morphology plaques, the ability to make preliminary comparisons of aortic 99mTc-tilmanocept uptake by SPECT/CT in each group, and to evaluate the ability of 99mTc-tilmanocept to identify the same aortic atherosclerotic plaques that are identified by contrast enhanced coronary computed tomography angiography (CCTA) and/or PET/CT.
For more information: www.navidea.com


 November 12, 2025
November 12, 2025