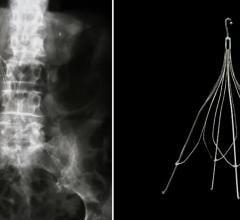
October 3, 2007 - Crux Biomedical Inc., a medical device firm, announced it recently completed the first implant in the United States of the Crux IVC Filter, as part of its strategy to deliver an inferior vena cava filter (IVC) that better meets physicians’ needs.
The filter is used to prevent pulmonary embolism (PE) and is designed to provide self-centering, ease of retrieval and low profile. Crux’s IVC filter was implanted at the Atlanta Medical Center by David Rosenthal, M.D., chief of Vascular Surgery. Dr. Rosenthal is the Principal Investigator for Crux’s pivotal IDE study, which is being conducted at 12 leading medical institutions in the United States.
Annual sales of IVC filter in the United States are estimated to be $252MM in 2008 and growing between 8-10% annually. Filters are increasingly being used as a result of increased diagnosis of deep vein thrombosis and the protection that they afford in cases of trauma and orthopedic surgeries.
For more information: www.cruxbiomedical.com


 July 27, 2021
July 27, 2021