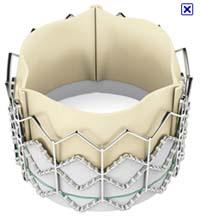
Edwards SAPIEN XT transcatheter aortic heart valve
March 2, 2010 — A new transcatheter valve and delivery systems has received European market clearance.
A disciplined European launch of the transcatheter aortic heart valve and the 18 French NovaFlex delivery systems is being planned, with the goal of expanding the number of commercial sites utilizing the systems throughout the year.
The Edwards SAPIEN XT transcatheter valve enables doctors to replace failing aortic valves without major surgery. The leaflet design of this new valve is modeled after Edwards' aortic tissue valves and its cobalt chromium frame provides improved radial strength and, therefore, enhanced circularity. The Edwards SAPIEN XT valve with the NovaFlex transfemoral delivery system is designed to provide easy, precise, balloon-expandable delivery of the valve.
As heart teams continue to gain clinical experience with the Edwards SAPIEN XT valve on the Ascendra 2 transapical delivery system, Edwards will begin introducing that system commercially in Europe during the second quarter. The Ascendra 2 system features a reduced profile and is designed for improving ease-of-use when delivering the valve through a small incision between the ribs.
The Edwards SAPIEN XT valve is the second commercially available transcatheter valve in the Edwards SAPIEN product portfolio. Edwards' transfemoral and transapical transcatheter aortic valve systems have been available in Europe since 2007. In the United States, the Edwards SAPIEN valve is an investigational device being studied as part of the world's only randomized, pivotal clinical trial of a transcatheter aortic valve and not yet available commercially.
For more information: www.edwards.com


 December 24, 2025
December 24, 2025 









