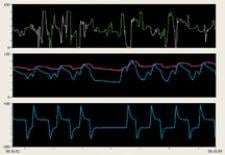
Top panel: ECG (green) Middle panel: FiberOptix Sensor arterial pressure (blue) fluid-filled arterial pressure (red) Bottom panel: balloon pressure waveform (blue)
Maintaining a continuous, accurate arterial pressure (AP) signal in the presence of electrosurgical interference is paramount to ensuring precise triggering and timing parameters. It has been demonstrated that heart failure patients on IABP therapy can experience timing errors during arrhythmias that can adversely affect left ventricular (LV) performance. Also, the delays and distortions of traditional fluid-filled transducer systems have been shown to interfere with timing, contributing to a misrepresentation of hemodynamics. Therefore, continued triggering of the pump and precise timing of balloon inflation in spite of irregular heartbeats are critical to effective IABP therapy in the OR environment.
Abstract
A 57-year-old patient presented with severe functional mitral regurgitation and advanced LV dysfunction (ejection fraction = 25 percent). The patient’s EuroSCORE was 7 and predicted mortality was eight percent. The patient underwent a mitral valve repair and was supported with intra-aortic balloon pump (IABP) therapy — using the AutoCAT 2 WAVE — during the procedure due to anticipated problems of arrhythmias and low cardiac output. Potential interference of adequate IABP function from other electrical devices used in patient care, such as pacer and cautery, were anticipated. The combined use of Arrow International’s FiberOptix Sensor Signal and AutoPilot mode of operation was able to overcome repeated electrical interference to delivery, providing timely and consistent IABP support, even during arrhythmic episodes.
IABP Response
During the procedure, the use of cautery caused interference with the ECG signal as a trigger source and the fluid-filled AP waveform continually dampened. The AutoPilot operational mode combined with the clear, precise AP signal from the FiberOptix IAB allowed consistent IABP operation to continue, and after the patient’s ECG was stabilized, AutoPilot returned to ECG triggering.
When cautery was used again later in the procedure, AutoPilot automatically switched back to AP trigger using the unaffected fiber optic AP signal. After bypass, high pacer output interfered with appropriate ECG signal recognition; adjustments to the pacer output and ECG gain allowed for consistent IABP support in the immediate postoperative period. The postoperative course continued to be uneventful and well supported until completion of IABP therapy. The patient was removed from IABP support after 48 hours, extubated and discharged from the ICU.
Successful Outcome
The combined use of AutoPilot mode of operation and FiberOptix IAB Sensor Signal was able to successfully overcome electrosurgical interference. The stability of the IABP support was maintained during the time of treatment, allowing a smooth postoperative course for the very high-risk patient.
The clinical benefits of improved timing and triggering accuracy for optimized IABP management are direct results of the fiber optic system’s ability to overcome interference associated with traditional fluid-filled AP signal acquisitions.
References:
1. Schreuder JJ, Maisano F, Donelli A, et al. Beat-to-beat effects of intraaortic balloon pump timing on left ventricular performance in patients with low ejection fraction. Ann Thorac Surg. 2005; 79:872-880.
2. Pantalos GM, Gillars KJ, Dowling RD, et al. Intraaortic balloon pump (IABP) timing errors in adult patients. ASAIO J. 2003; 49:155.
3. Nashef SA, Roques F, Michel P, et al. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg. 1999; 16:9-13.



 August 14, 2023
August 14, 2023 









