Image from presentation, "The PHARMCLO Study," Diego Ardissino, M.D., F.A.C.C.
March 20, 2018 — Patients with acute coronary syndrome experienced a substantially lower rate of heart attack, stroke, death from cardiovascular causes and major bleeding at 12 months if genetic information was used to inform the selection of their antiplatelet medication in a study presented at the American College of Cardiology’s 67th Annual Scientific Session.
Acute coronary syndrome is an umbrella term that encompasses heart attacks and unstable angina (acute chest pain caused by narrowed arteries). To reduce the risk of future ischemic events (caused by thrombosis, the blockage of a blood vessel by a blood clot), patients with acute coronary syndrome are prescribed a daily aspirin and one of several antiplatelet medications in a class known as P2Y12 receptor antagonists.
Three main P2Y12 receptor antagonists are available: clopidogrel, ticagrelor and prasugrel. In deciding which one to use, doctors weigh a patient’s risk of ischemic events against the risk of uncontrolled bleeding. Previous research has indicated that patients’ genes can affect how well these different drugs work in individual patients.
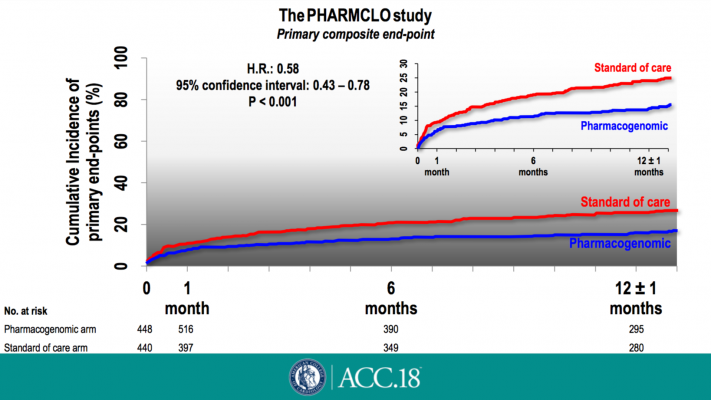
The study, called PHARMCLO, is the first to combine clinical characteristics with genetic information to inform the choice of P2Y12 receptor antagonist in patients with acute coronary syndrome. At 12 months, patients who received a genetic test to inform the choice of medication were 42 percent less likely to experience heart attack, stroke or death from cardiovascular causes, or major bleeding (the trial’s composite primary endpoint) compared with patients who did not receive the genetic test.
“Selecting treatment on the basis of genetic data in addition to considerations concerning the patients’ clinical characteristics may lead to a more personalized, and therefore more efficient, antiplatelet therapy, thus reducing both ischemic and bleeding risk,” said Diego Ardissino, M.D., cardiologist at Azienda Ospedaliero-Universitaria di Parma, Italy, and the study’s lead author. “PHARMCLO is the first step of a new approach that will see a shift in emphasis away from trying to discover ever more potent antithrombotic drugs and toward ensuring that the right therapy is given to each individual patient.”
Researchers enrolled 888 patients hospitalized for acute coronary syndrome in Italy. Half were randomly assigned to receive standard clinical care, in which doctors prescribed clopidogrel, ticagrelor or prasugrel based on the patient’s clinical characteristics alone. The other half were assigned to receive a genetic test, the results of which doctors considered, along with clinical characteristics, when prescribing antiplatelet therapy. At 12 months, the trial’s composite primary endpoint had occurred in 25.9 percent of patients receiving standard care and 15.8 percent of patients receiving the genetic test.
Genetic testing also resulted in different prescribing patterns. While prasugrel was prescribed at similar rates in both groups, clopidogrel was prescribed significantly more frequently among those who did not receive a genetic test and ticagrelor was prescribed significantly more frequently among those who did receive a genetic test. Previous studies have shown prasugrel and ticagrelor to be superior to clopidogrel at preventing ischemic events. However, prasugrel and ticagrelor, which are more potent, are also known to increase the risk of bleeding. The findings suggest that having more information about a specific patient’s likely response to clopidogrel can help doctors weigh this trade-off.
Several genes have been shown to affect enzymes that make clopidogrel more or less effective in preventing platelet aggregation. For this study, researchers developed an easy-to-use genetic screening tool, ST Q3, that provides information about these genes from a blood sample in just 70 minutes.
“As genotyping to select P2Y12 receptor antagonists in the setting of acute coronary syndromes cannot be delegated to centralized genetic laboratories for reasons of time, we designed the ST Q3 instrument for bedside genotyping as a low-cost, portable system for foolproof use by unskilled personnel,” Ardissino said.
Researchers suggest the study findings offer a proof of concept that personalized genetic information can be used to inform treatment decisions and improve outcomes for people with acute coronary syndrome. Further research would be needed to confirm the findings and incorporate genotyping into clinical practice for this patient population. The PHARMCLO study stopped recruiting patients early after the Ethics Committee of Modena (Italy) required the trial to be prematurely stopped due to the lack of in vitro diagnosis (IVD) certification for the ST Q3 instrument. All of the patients were followed up as planned.
For more information: www.acc.org


 July 31, 2024
July 31, 2024 









