July 23, 2010 – For patients suffering from a heart attack, every minute counts, yet the average time from start of chest pain to receiving emergency room (ER) medical attention is 3.2 hours. The average turn-around time of lab-based test results is 2.8 hours, with the minimum time to detect a heart attack using today’s lab-based immunodiagnostics is 4.5 hours from symptom outset.
When it comes to cardiac events and survival, the key factors for a successful recovery are time to diagnosis, accuracy of diagnosis, and appropriate clinical management. For these reasons point-of-care (POC) testing for chest pain patients may help improve patient survival.
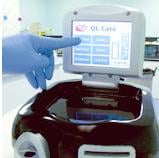
Ontario-based CardioGenics has created improved cardiac diagnostic testing that boasts faster results, better accuracy, at reduced cost to the patient and caregiver. The analyzer offers advantages over existing POC immunoassay technology, which the company compares to the shift from portable CD players to iPods. In six steps, the QL Care Analyzer provides printed medical lab quality results in less than 15 minutes. Capable of delivering test results in the ER, doctor’s office, or EMS vehicle, the single-use cartridge QL Care Analyzer is the size of a portable CD player, features an intuitive user interface touch screen, and is capable of storing patient data for up to 5,000 individuals.
The QL Care Analyzer is being submitted to the U.S. Food and Drug Administration (FDA) this summer for testing of troponin I, a protein that is integral to cardiac muscle and its blood level measures the presence and severity of a heart attack.
CardioGenics believes that the device cartridge can ultimately be adapted to run any number of immunoassay tests. (There are approximately 200 different immunoassays for HIV, cancer, thyroid conditions, etc., that are currently performed by lab-based analyzers).
For more information: www.cardiogenics.com


 October 09, 2019
October 09, 2019 









