June 30, 2017 — Abiomed Inc. announced the recent publication of a peer-reviewed retrospective study on hemodynamic support with the Impella 2.5 heart pump. The study analyzed patients with acute myocardial infarction complicated by cardiogenic shock (AMICS) undergoing a percutaneous coronary intervention (PCI) on an unprotected left main coronary artery (ULMCA). The study was published in the Journal of Interventional Cardiology.
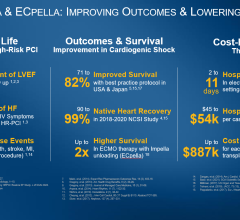
Results of the study suggest that initiation of Impella 2.5 heart pump prior to the start of a PCI on an ULMCA culprit lesion is associated with significant survival benefit in patients supported for cardiogenic shock following an AMI. Patients supported with the Impella 2.5 heart pump post-PCI appear to have very poor survival at 30 days. This study adds to the existing body of clinical data supporting the early placement of Impella heart pumps before PCI for patients in cardiogenic shock.
A heart attack in the left main is sometimes referred to as the “mother of all widow makers” because it carries a mortality rate of over 80 percent. The left main coronary artery and its branches supply oxygenated blood to 75 percent of the heart’s left ventricular muscle mass. This important artery is the only source of blood and oxygen to the left side of the heart, thereby deemed ‘unprotected’ when there is no history of heart surgery to bypass the left main coronary artery.
Perwaiz Meraj, M.D., and colleagues reported for the first time the real-world outcomes of Impella 2.5 use in PCI on a ULMCA culprit lesion in patients with AMICS. This multicenter, retrospective study included 36 patients from 19 U.S. sites participating in the cVAD Registry in order to assess whether the initiation of hemodynamic support before PCI would have a survival benefit compared to initiation of support after the PCI. The majority of these patients (average age 69) were in cardiogenic shock at the time of hospital admission (73 percent) and had a low mean ejection fraction of 25 percent.
The study demonstrated a significant hospital survival benefit when Impella 2.5 was initiated prior to PCI:
| Impella pre-PCI (n=20) | Impella after PCI (n=16) | p Value | |
| Survival to Discharge | 55% | 19% | 0.041 |
| Survival at 30 Days | 48% | 13% | 0.004 |
“Patients with cardiogenic shock complicating an acute myocardial infarction due to an unprotected left main coronary artery culprit lesion are some of the sickest and most clinically challenging patients admitted in the cath lab,” said Meraj, of Northwell Health and first author of the manuscript. “Our data suggests that early placement of Impella before PCI is vital to survival.”
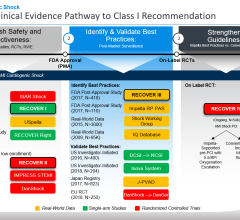
These new data support prior publications with percutaneous heart pumps (such as Impella) supporting cardiogenic shock patients published in Journal of the American College of Cardiology (National trends in the utilization of short-term mechanical circulatory support) and the Journal of Interventional Cardiology (Use of Impella 2.5 in Acute Myocardial Infarction complicated by Cardiogenic Shock) representing nearly 12,000 Medicare/insurance patients and 154 cVAD Registry patients respectively. Additional data from Abiomed's observational IQ Database of 15,259 AMICS patients and data from the Detroit Cardiogenic Shock Initiative reinforce the best practice of placing Impella heart pump before PCI in cardiogenic shock patients.
Read the article "Improving Cardiogenic Shock Survival With New Protocols."
For more information: www.protectedpci.com


 April 28, 2023
April 28, 2023